|
Binu
sasidharan 1, Prem Kotian 2, Jagannath Kamath B 3, Deepak Jayaram
4, Krishnaprasad 5, Vandana Vamadevan 6, Muralikrishnan,
Kannampillil 7, Vijay Tubaki 8, Vishal Mangrolia 9, Anup
Kumar 10
1.
Senior Resident, Dept. of Orthopaedics,
2. Professor, Dept. of Orthopaedics,
3. Professor,Dept. of Orthopaedics,
4. Specialist Registrar, General internal
medicine, Lancaster Royal Infirmary,
Lancaster,United Kingdom.
5. Associate professor, Dept of Medical
Oncology,
6. Junior resident, Dept of Otorhinolaryngology
and head and neck surgery,
7. Final MBBS Student
8. Consultant Orthopaedic Surgeon, Unity Hospital,
Mangalore.
9. Consultant orthopaedic surgeon, G T Sheth
orthopaedic hospital, Rajkot, Gujarat.
10. Assistant professor, Dept. of orthopaedics, Kasturba Medical College, Mangalore.
Address for Correspondence:
Binu sasidharan,
Kottarathil, No.37, Maithrinagar,
Kottarakkara, Kollam, Kerala.
Email: binunirmal2000@yahoo.com
Tele: 09845800912
|
|
J.Orthopaedics 2008;5(1)e19
Introduction:
Ever since William McIntyre 1
gave the first description of multiple myeloma(MM) in 1850,this
disease has been an area of constant research because of the
plethora of symptoms by which the disease can present, lack of
effective treatment and poor prognosis.Multiple myeloma which
accounts for 1% of all malignancies represents an unrestrained
proliferation of a single clone of plasma cells leading to
increased production of immunoglobins which are structurely
abnormal and functionally incompetent. Last four decades has
seen great progress in understanding the diagnosis and treatment
of this deadly condition.A major breakthrough happened in 1958
when Blokhin of Russia reported the successful use of a racemic
mixture of D and L phenylalanine mustards (Melphalan) in the
management of myeloma
1.
The most widely used staging system in multiple myeloma
is the Durie Salmon staging 2which
was introduced in 1975.In 2003, Griepp et al proposed a new
staging system named as International Staging System(ISS) 3,4
which is a simpler one.In our study we used this new staging
system and analyzed the significance of various
parameters in predicing therapeutic response in myeloma.
Material and Methods :
This is a prospective study that includes 35 newly
daignosed cases of symptomatic myeloma
treated in our institution
between January 2005 and April 2007. Permissionto conduct the study was taken from local
ethical committee.We used the diagnostic criteria proposed by
International myeloma working group 2003 4,5(Table
1).
Patients
who were not willing (financial constraints) or not
eligible(age, medical contraindications) to receive high dose
chemotherapy and autologous stem cell transplantation (SCT) were
included. Patients with non secretory myeloma, monoclonal
gammopathy of unknown significance, smouldering myeloma and
those patients who were willing/eligible to undergo autologous
SCT and those with a serum creatinine >3 mg/L after adequate
hydration were excluded from the study .Patients with associated
hepatic, cardiac or pulmonary disease and the immunocompromised
were also excluded.
Table 1
==================================================
 Diagnostic
criteria for symptomatic multiple myeloma (International Myeloma
Working Group,2003) Diagnostic
criteria for symptomatic multiple myeloma (International Myeloma
Working Group,2003)
*M
paraprotein in serum and/or urine.
**Bone marrow (clonal) plasma cells or
plasmacytoma.
Related
organ or tissue impairment (ROTI)(end organ damage) which is
manifested by
a)
Increased calcium levels
b)
Renal insufficiency
c)
Anemia
d)
Bone lesions
e)
Others: symptomatic hyperviscosity,amylodosis and recurrent
bacterial infections
*No
specific level of serum or urine M protein is included in this
diagnostic criteria.
**Similarly no minimal level of bone marrow
plasma cells was designated.
==================================================
Prospective evaluation included collection
of demographic, clinical, laboratory,radiographic, treatment and
follow up data.Various clinical presentations were noticed and
recorded. Investigations
included routine blood examination, platelet count, peripheral
smear, C reactive protein (CRP), lactate dehydrogenase (LDH),
serum albumin, globulin, urea, creatinine, serum calcium, serum
β2 microglobulin, uric acid, Bence Jones Proteins (BJP),
serum and urine electrophoresis and bone marrow aspiration.
Electrophoresis of serum and
concentrated urine was performed in all cases.We used agarose
gel electrophoresis to screen for the presence of M
proteins.Quantification of M component was performed by
densitometry of the monoclonal peak on
electrophoresis.Immunofixation was performed when multiple
myeloma or related disorders were suspected despite a normal
serum electrophoretic pattern
6.It was also used to confirm a complete response
following chemotherapy.
Radiological evaluation included
radiographs of the specific areas where the patient complained
of bone pain, swellings or pathological fractures.When multiple
myeloma was suspected skeletal
imaging survey was done.Computerized tomography and magnetic
resonance imaging were done in selected cases.
All the 35 patients in this study were categorized into
three stages based on International Staging System(ISS)
3 and British Medical Research Council (BMRC) staging 7.Durie Salmon staging requires M component production
rates to categorize the patients
2.This staging was not done because of incomplete
data.
The new ISS put forward by Greipp et al in 2003 is based
on two easily measurable parameters, serum albumin and β2
microglobulin (β2 M) (Table 2).
Table 2
International Staging System (Greipp et
al, 2003)
|
Stage
|
Findings
|
Prognosis
|
|
I
|
β2 M < 3.5
Albumin ≥ 3.5
|
Good
|
|
II
|
Neither stage I nor stage III
β2 M < 3.5 or
β2 M-3.5 to 5.5
Albumin ≥ 3.5
|
Intermediate
|
|
III
|
β2 M ≥ 5.5
|
Poor
|
Patients with impaired renal function
carries a bad prognosis irrespective of the stage.
The BMRC staging is based on haemoglobin,
blood urea and the level of activity which is determined by
Karnofskys performance status scale (Table 3).
Table 3
British Medical Research Council Staging
|
Stage
|
Findings
|
Prognosis
|
|
A
|
Hb >10 gm%
Blood urea <22mg%(8 mmol/L)
Minimal symptoms (karnofsky 100)
|
Good
|
|
B
|
Neither A nor C
|
Intermediate
|
|
C
|
Hb < 7.5 gm%
Blood urea > 28mg%
Restricted activity (karnofsky <
70)
|
Poor
|
Treatment :
General supportive measures undertaken
included correction of anemia and metabolic abnormalities
,maintanance of renal function and adequate hydration,
appropriate antibiotics to treat infections and paracetamol and
weak opioids like codeine for pain relief.Allopurinol was given
in 12 cases where serum uric acid levels were
elevated.Bisphosphonate therapy was administered in all
cases.Zoledronic acid 4 mg i.v was given monthly for minimum 12
months or death whichever is earlier.Creatinine levels were
checked before each zoledronic acid infusion.Pamidronate was
used at a slower infusion rate in two patients with borderline
renal function. Appropriate spinal braces were used for stable
compression fractures.
Twentyfive patients were treated with chemotherapy alone,
five patients with
surgery ,chemotherapy and radiotherapy and five patients with
chemotherapy and
radiotherapy.Chemotherapy consisted of oral melphalan (8 mg/m2
) on days 1-4 and oral
prednisolone (60
mg/m2 ) on days 1-4.The cycles were repeated every 28
days.We usually give 12 cycles or until intolerance or disease
progression was observed.
Respose
assessment
We assessed the therapeutic response before starting the
fourth cycle of chemotherapy using EBMT response
criteria 4
which is given below:
* EBMT-European group for blood and marrow
transplantation
IBMTR-International bone marrow transplant registry
ABMTR-Autologous blood and marrow transplant registry
Types of responses:
- Complete response (CR)--No M protein is detected
in serum or urine by immunofixation for a minimum of 6 weeks and less than 5%
plasma cells in bone
marrow.
- Partial response--more than 50% reduction in serum
M protein and/or
90% reduction in urine
free light chain excretion.
- Minimal response—25-49% reduction in serum M
protein level and/or
50-89% reduction in
urine free light chain excretion.
- Plateau—No evidence of continuing myeloma
related organ or tissue damage, less
than 25% change in M protein levels and light chain excretion
for 3 months.
- Progresive disease—Myeloma related organ or
tissue damage continuing despite therapy.
- Relapse—Reappearance of disease in patients previously
in CR.
Responders
and nonresponders were statistically analysed to assess the
significance of various parameters in predicting response to
therapy.The factors that were analyzed included age, gender,
haemoglobin level, ESR, platelet count, blood urea,
serum creatinine, serum albumin, calcium, b2
microglobin, LDH, CRP, Bence Jones proteins and percentage of
bone marrow plasma cells .Statistical
analysis was done using version 14 of SPSS data editor
software.Different categories of each parameter were compared
using p-value and the level of significance was set at p <
0.05.
Results :
The patients included 18 males and 17
females with a mean age of 58.8 years at the time of
presentation.There were 2 cases with age less than 40 years.
Clinical spectrum
The various clinical presentations in our study are
depicted in the bar chart (Fig 1). Bone
pain was the commonest presenting symptom in our study( 83%).The
commonest site of bone pain was over spine(60%) followed by
pelvis and ribs.Pathological fractures were present in 21
patients.Out of this 21 patients ,vertebral compression
fractures were present in 18 ,fracture shaft of humerus in 1
patient,fracture shaft of femur in 1 and intertrochanteric
fracture in 1 patient.Of the 7 patients presented with
infections, 3 had pnuemonia, 2 had urinary tract infection, one
had cellulitis, and one had maxillary sinusitis.Two
patients presented with paraparesis and one patient presented
with sensory deficits. One patient presented with generalized
tonic clonic seizures which on work up turned out to be multiple
myeloma. CT images of this patient are shown in fig 2. Of
special interest is
a patient who presented with sensory deficits in both
lowerlimbs, hyperpigmentation of tongue and skin and bone
pains.On furthur evaluation, prostatomegaly and diabetes
mellitus were detected and POEMS syndrome8
was diagnosed.
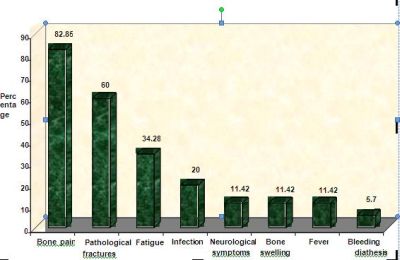
Fig 1
Fig 2
Investigations
The results of various investigations are listed below:
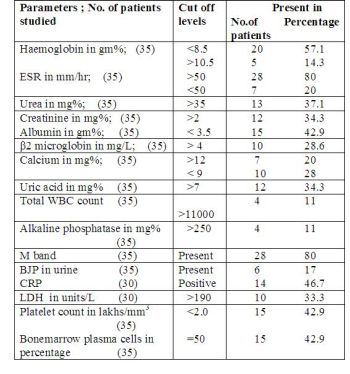
77% of patients were anaemic at presentation.Anaemia was
normocytic normochromic variety in majority of cases.One
interesting observation was that the number of patients who were
hypocalcemic outnumbered those who were hypercalcemic.Elevated
alkaline phosphatase seen in 4 patients may be attributed to
pathological fractures.
Radiological evaluation revealed that 4
patients had solitary plasmacytomas,29 had multiple osteolytic
lesions,1 had osteosclerotic lesion and 1 had osteoporosis
only.The osteosclerotic lesion of the vertebra was seen in the
patient with POEMS
syndrome.
Staging
When ISS was applied, 17 patients come under stage
І, 12
patients in stage ІІ
and
6 in stage ІІІ.This implies good prognosis in
17 patients, intermediate prognosis
in 12 and poor prognosis in 6. When BMRC staging was applied, 5 patients come
under stage A, 22 patients in
stage B and 8 in stage C.
Treatment
None of the patients developed
serious complications following chemotherapy. Eight patients on
melphalan developed alopecia, three developed diarrhea and
gastro intestinal upsets, two had febrile reaction and one had
hyperpigmentation of skin. Haematological toxicity was limited
to grades 1 and 2.
Surgery: One patient had fracture shaft
of femur, one had intertrochanteric fracture and one had
fracture shaft of humerus.Open reduction and internal fixation
was done in these 3 cases.The patient operated for
intertrochanteric fracture developed addisonian crisis following
surgery,but survived. Two patients had motor weakness from
spinal cord compression ( one by plasmacytoma and other one by
pathological compression fracture ) and they were treated by
spinal decompression followed by radiotherapy and chemotherapy.
Radiographs of a case where internal fixation was done for
pathological fracture
is shown in fig.3.
Radiotherapy: Radiotherapy was
given post operatively in 5 cases and in 5 cases of solitary
plasmacytomas.In all these cases chemotherapy was started prior
to radiotherapy.
Response
assessment
According
to CLMTF criteria9
there are three types of responses—objective response, partial
response and treatment failure.Here complete remission was
considered as a subset of objective response.We considered
complete response and partial response in EBMT criteria as an
objective response.Plateau phase and progressive disease are
grouped as treatment failure. Response assessment using EBMT
criteria showed that 3 patients had complete response,15 had
partial response, 9 had minimal response, 5 were in plateau
phase and one had progressive disease.(Two patients defaulted
after two cycles of chemotherapy).Altogether there are 18
objective responses,9 minimal responses and 6 treatment
failure.This shows remarkable correlation with ISS.( stage
I-17,stage II-12 and stage III-6). BMRC staging doesnot show
such correlation.
Of the five patients who expired during the course of our
study,three were defaulters during various stages of
chemotherapy and two were getting chemotherapy at the time of
death. Two patients were lost on follow up after six cycles of
chemotherapy.
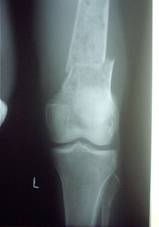
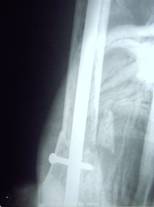
Fig.3a
Fig.3b
Analysis of p
values showed that serum albumin (p=0.001), β2 microglobin
(p=0.001), LDH (p=0.001), CRP(p=0.001), urea (p=0.002),
creatitine (p=0.001), platelet count (p=0.014) and bonemarrow
plasma cells (p=0.014) are statistically significant parameters.
Discussion:
Lack
of a uniform criteria for diagnosis and response assessment
posed problems in interpreting results of various myeloma study
groups. A wide range of diagnostic criteria have been used by
various investigators like Medical Research Council (MRC) of the
United Kingdom, Nordic Myeloma Study Group,Chronic
Leukemia-Myeloma Task Force (CLMTF) of the National Cancer
Institute, Eastern Cooperative Oncology Group (ECOG) and
South West Oncology Group ( SWOG)
.In 2003 the International myeloma working group has
proposed a uniform diagnostic criteria which helps to
standardize the results of various study groups.
Several
criteria for evaluation of therapeutic response are currently in
use. CLMTF criteria, SWOG criteria and the widely used * EBMT/ IBMTR/ ABMTR response criteria (commonly
referred to as EBMT criteria) are some of them. Recently in 2006
Durie and his associates modified the EBMT criteria and proposed
an international uniform response criteria for multiple myeloma
10.Response to chemotherapy was assessed before
starting the fourth cycle of chemotherapy since investigators
like Belch et al reported that a minimum period of 3 months is
required to get an effect out of chemotherapy.11
Studies done to assess the relationship
between a positive
therapeutic response and survival yielded contrasting results in
the past due to various reasons.Majority of such studies were
retrospective,different response criteria were used,time to
response were different and dosing schedules varied.Schaar et al
12 (2004)
prospectively assessed the relationship between survival and the
rate of monoclonal protein (M-protein) decrement during the
first cycles of chemotherapy in 262 patients with newly diagnosed MM and concluded
that early response to chemotherapy predicts for survival in
MM.In another
prospective study Powels et al showed that therapeutic response
predicts for survival.Retrospective studies by Tsuchiya et al
(1994) 13
and Blade J et al (1998)9 showed that a good response to therapy is associated
with a longer survival.Recently Pineda Roman et al (2007)
observed that complete response in myeloma extends survival in
patients with no history of MGUS or smouldering myeloma 14.Hence therapeutic response can be taken as a
surrogate marker of survival.It needs to be furthur observed
whether patients showing objective response to chemotherapy in
this study survives longer.
That forms the future perspective of this study.
High dose
chemotherapy followed by stem cell rescue has become a standard
therapy for patients younger than 65 years.But advanced age,
comorbidities and financial constraints often preclude the use
of this approach and conservative regimens become the
cornerstone of therapy for such patients.Melphalan-prednisolone
regimen (MP) has been the gold standard therapy for multiple
myeloma for many years.Although VAD
regimen (Vincristine, Adriamycin and Dexamethasone) has a
rapid and superior objective response owing to the increased
magnitude of tumour cytoreduction, it has not shown to prolong
overall survival15,16.The
administration of VAD regimen requires a central venous
catheter, which leads to an appreciable incidence of sepsis and
thrombosis4.However VAD regimen do not require dose adjustment in
renal failure.A meta-analysis by myeloma trialists’
collaborative group(1998) confirmed that MP was as effective as
combination chemothearapy ,and was more conveinient because of
its oral route of administration and lower cost16.Only
patients treated with MP were included in our study due to the
variation in the magnitude of response with MP and VAD regimen..
There are reports confirming the efficacy of thalidomide and
dexamethasone as first line therapy for newly diagnosed myeloma17.We
used thalidomide
only for refractory and relapse cases. Thalidomide can be used
in newly diagnosed myeloma patients only in the context of a
clinical trial.4.Lenalidomide
and bortezomib were not used in any of our patients.
Bone
pain was the commonest symptom in our series.This coincides with
the studies of Kyle RA (2003) 18 and Advani et al (1978) 19.The next common presentation in our study is
pathological fractures(60%). 20% of our patients presented with
infections.Kyle RA reported similar figures in his series .Bone
swellings were noticed in 11% of patients in our series,whereas
Advani et al reported an incidence of 23%. No extramedullary
plasmacytomas were noted in our study.
Patients with compromised renal
function showed poor response to chemotherapy.This was confirmed
in many previous studies 9,13,18,19
.Patients with elevated serum LDH and positive CRP had a poor
therapeutic response.This correlates with the current body of
literature 20,21.CRP assay is proposed as a surrogate for
measurement of IL-6 levels21,22.
Thrombocytopenia also predicts a poor therapeutic
response(p=0.014)18.Bence
Jones Proteins were detected in urine in 17% of our patients.The
reported figures in literature ranges from 9% to 77%.Several
studies have shown that presence of BJP is associated with poor
survival.The observations in our study shows that presence of
BJP does not have any significance in predicting therapeutic
response.This coincides with the findings of Advani et al19.
Normal β2 microglobin is 1.6 ±
0.4 mg/L.There is an excellent correlation between serum β2
M levels and myeloma tumor burden.Twenty eight percentage of our
patients had β2 microglobin more than 4 mg/L.High levels of
serum β2 microglobin and low albumin were associated with
poor therapeutic response (p= 0.001).This correlates with the
observations of several myeloma working groups3.
However Advani’s study doesnot show any difference in survival
with respect to albumin level.
A large number of bonemarrow plasma cells (>50%) is
representative of high tumor load and is associated with a poor
therapeutic response. (p-0.014). Studies by Tsuchiya
et al 13
and Advani et al 19
showed that a low plasma cell percentage in bonemarrow is
associated with longer survival.The significance of
plasmablastic morphology was not analyzed in the current study.
Staging
A good staging system enables the physician to categorize
the patients according to risks and plan the management. It
helps in predicting therapeutic outcome and survival.It also
facilitates comparison between clinical trials.Durie Salmon
staging is the most widely used staging system for multiple
myeloma since its introduction in 1975. It classifies myeloma
into 3 stages based on haemoglobin, serum calcium levels, bone
lesions on X rays, M component
production rate and
serum creatinine.This staging has several disadvantages.
-
Evaluation of bone lesions by X rays carries an element
of interobserver variation.
-
Determination of M component production rate
requires identification of immunoglobin types by immunofixation
.
-
When Durie Salmon staging was used majority of
patients come under stage 3.This causes problems in predicting
therapeutic response and survival in low risk and intermediate
risk patients.
-
The criteriae are complex and many laboratory
parameters are required.
The disadvantage noticed with BMRC staging in our
study is that a large subset of patients come under intermediate
stage.The new ISS is simpler and it requires only two
parameters, serum β2 microglobin and albumin.Both
parameters are quantitative in nature.Moreover this staging
system incorporates serum β2 microglobin which is probably
the best independent prognostic indicator for multiple myeloma
identified so far.
Conclusions:
The
new International Staging System shows good correlation with
therapeutic response and it is simpler than conventional staging
systems.Low serum albumin, high β2 microglobin, high urea,
high creatinine, low platelet count,
large number of bonemarrow plasma cells , positive CRP and high
serum LDH are
associated with poor therapeutic response.
Acknowledgements:
We are grateful to Kotian MS for compiling the data
and statistics. We thank Dr.Sijeesh and Dr.Vishesh Kothari, Dept
of internal medicine for their valuable
advice, discussion and
input.
Reference :
- Kyle RA. Multiple myeloma: An odyssey of discovery.Br J Haematol
2000;111:1035-44.
- Durie BGM, Salmon SE. A clinical staging system for multiple
myeloma:correlation of measured myeloma cell mass with
presenting clinical features,response to treatment,and
survival.Cancer 1975;36:842-854.
- Griepp PR, Miguel JS,Durie BGM,CrowleyJ.International Staging
System for multiple myeloma. J Clin Onco 2005;23:3412-3420.
- Smith A, Wisloff F, Samson D.Guidelines on the diagnosis and
management of multiple myeloma. Br J Haematol 2005;132:410-451.
- Kyle RA. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report
of the International Myeloma Working Group British Journal of
Haematology 2003;121:749–757.
- Kyle RA. Sequence of Testing for Monoclonal Gammopathies,Serum and Urine Assays. Archives
of Pathology and Laboratory Medicine 1999;123:114–118.
- Gassmann W, Pralle H. Staging systems
for multiple myeloma: a comparison. Br J Haematol
1985;59:703-711.
- Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV,
Therneau TM, Larson DR. POEMS syndrome: definitions and
long-term outcome.Blood 2003;101:2496-2506.
- Blade J, Fernandex-Llama P, et al. Renal
failure in multiple myeloma. Presenting features
and predictors of outcome in 94 patients from a single
institution. Arch Intern Med 1998;158:1889-1893
- Durie BGM, Harousseau JL, Miguel JS, Blade J, Barlogie B,
Anderson A International uniform response criteria for
multiple myeloma.Leukemia 2006;20:1467–1473.
- Belch A, Shelley W, Bergsagel D, Wilson K, Klimo
P,White D, Willan A. A randomized trial of
maintenance versus no maintenance melphalan and prednisolone in
responding multiple myeloma patients.British Journal of Cancer
1988;57: 94-99.
- Schaar CG, Kluin-Nelemans JC, Cessie S.Early response
to therapy and survival in multiple myeloma.Br J Haematol
2004;125:162-166.
- Tsuchiya J, Murakami H, Kanoh T :Ten year
survival and prognostic factors inMultiple myeloma.Br J
Haematol 1994;87:832-834.
- Pineda Roman M,Bolejack V,Arzoumanian V,Frits van Rhee,Zangari
M,Walker R. Complete response in myeloma extends survival
without, but not with, history of prior monoclonal gammopathy of
undetermined significance or smouldering disease. Br J Haematol
2007;136:393-399.
- Hjorth M,
Hellquist L, Holmberg E, Magnusson B,Rodjer S, Westin J.Initial
treatment in multiple myeloma: No advantage of multidrug
chemotherapy over melphalan-prednisone.The Myeloma Group of
Western Sweden.Br J Haematol 1990;74:185-91.
- Myeloma
Trialists’ Collaborative Group.Combination chemotherapy versus
melphalan plus prednisone as treatment for multiple myeloma: An
overview of 6633 patients from 27 randomized trials.J Clin Oncol
1998;16:3832-42.
- Rajkumar SV,
Hayman S, Gertz MA,Dispenzieri A, Lacy MQ, Greipp PR et al.
Combination therapy with thalidomide plus dexamethasone for
newly diagnosed myeloma. J Clin Oncol 2002;20:4319-23.
- Kyle RA.Review of 1027 patients with newly diagnosed
multiple myeloma. Mayo Clin Proceedings 2003;78:21-23.
- Advani SH, Soman CS, Talwalkar GV, Iyer YS, Bhatia
HM.Multiple myeloma: Review of 231 cases. Indian Journal of
Cancer 1978;
15:55
-61
- Dimopoulos MA. High serum lactic dehydrogenase level as a marker
for drug resistance and
short survival in multiple myeloma. Ann Intern Med
1991;115:931-35.
- Bataille R, Boccadoro M, Durie BGM. C-reactive protein and
beta-2-microglobulin produce a simple and
powerful myeloma staging system. Blood 1992;80:733-737.
- Rajkumar SV, Greipp PR.Prognostic factors in multiple myeloma.
Hematol Oncol Clin North Am 1999;13:1295-1314.
|





