|
Abstract:
Objective:
Seed cell
was the most important factor restricting the clinical
application of meniscal tissue engineering. Bone mesenchymal
stem cells(MSCs)which
has its own advantages become the potential cell source of
tissue engineering.
The arms of the study is to investigate the induction effect of
MSCs by meniscal cells under co-colutured without contact.
Methods:
MSCs and meniscal cells were isolated from bone marrow and meniscus
MSCs and meniscal cells were cultured in the either side of
membrane of Millopore’s hanging cell culture insert speratedly
and lasted for 7 days without different kinds of cells
contact.(non-contact co-culture and cells ratio of the
co-culture is 1:1). Immunohistochemistry and RQ-PCR was to
applied.
Result: Immunohistochemistry of type
Ⅰ
collagen of induced MSCs was positive and that of
Ⅲ
collagen was negative. mRNA expression of aggrecan type I and
III collagen were increased and that of type II collagen was not
increased in MSCs after induced by meniscal cell
Conclusion: MSCs which induced by meniscal cells with
non-contact co-culture method have differentiation potential to
meniscal cells. MSCs which induced by meniscal cells with
non-contact co-culture method may be the seed cells of tissue
engineering of the meniscus.
J.Orthopaedics 2010;7(4)e9
Keywords:
tissue engineering; meniscus; seed cell; bone marrow mesenchymal
stem cells
Introduction:
Background
Meniscal function is essential to the normal function of the
knee joint, but meniscal injuries are very common, especially in
athletes. (1)
Due to the lack of vasculature in the fibrocartilage, such as
the inner 1/3 portion of meniscus, injuries to them usually
result in formation of fibrous tissue which greatly alters joint
function and predisposes the joint to degenerative changes.
Surgical interventions like partial and total meniscectomy,
allogous meniscal transplantation, meniscal repairing and so on,
from some studies, have their relative limitations, such as
osteoarthritis, immunogenicity, disease transmission, limited
availability, limited indications.(2-8) Tissue
engineering may offer new treatment modalities for the
regeneration of meniscus lesions or for the complete replacement
of a degenerated or injured (part of the) meniscus by a
tissue-engineered meniscus. Tissue engineering is based on a
smart and unique combination of cells, growth factors and
scaffolds. The seed cell is a very important part of
tissue-engineered meniscus.
The meniscal cells from the patients which are excellent cell
source for a tissue-engineered meniscus seem to be less than
ideal for expansion or production sufficient matrix.(8,9)Mesenchymal
stem cells (MSCs)
are pluripotent cells present in many adult mesenchymal tissues,
such as synovium, muscle, adipose tissue and bone marrow and
usually isolated from bone marrow.(10) Bone marrow
mesenchymal stem cells(MSCs) will maintain cell morphology and
potent of multi-differentiation when culture in vitro.(11)
In exchange for a more painful harvesting technique, human bone
marrow mesenchymal stem cells (MSCs) may offer significant
advantages in terms of great proliferative capacity and
potential for differentiation toward fibrocartilaginous
lineages. (8, 9)
Hence, in this study, the induction effect of MSCs by meniscal
cells was investigated under co-colutured without contact.
Materials
and Methods:
The research protocol of this experiment was reviewed and
approved by the ethical committee of Sichuan University.
1. Meniscal cells source and culture
The New Zealand white rabbit were purchased from West China
experimental animal station of Sichuan University. For
4-week-old rabbits were anesthetized by an intraperitoneal
injection of sodium pentobarbital. Menisci were taken from the
knee joint and the meniscal cells were isolated and harvested,
according to previous studies. (12,13) After
removing soft tissue and slicing meniscus(1mm3),
the isolation starts with a short digestion in 0.05%
hyaluronidase for 5 min and a subsequent digestion in 0.2%
trypsin for 30 min, followed by the regularovernight incubation
in 0.2% collagenase type I . The digested tissue/cell suspension
was passed through 200μm cell strainer to remove tissue debris
and cells centrifuged. Cells were cultured in 1800rpm for 5 min.
The cells were suspended in culture media consisting of
Dulbecco’s modified Eagle medium and
Ham's
F12
(DMEM/F12 1:1) with 10% fetal bovine serum (Hyclone, USA),
100U/ml penicillin and 100μg/ml streptomycin. The cells were put
in a humid atmosphere containing 5% CO2, with medium changed
first 6 days and every 3 days thereafter.
2. Bone marrow mesenchymal stem cells source and culture
2ml of MSCs was aspirated from each tibia of rabbits mentioned
above with a 5ml syringe containing 0.2 ml heparin(1000 Unit per
ml). Mononucleated cells were isolated using a Histopaque-1090 (Haoyang,China)
density gradient method. These cells were cultured in a 75 cm2
flask with Dulbecco’s modified Eagle medium- low glucose (L-DMEM)( Gibco,
USA )supplemented with 10% fetal bovine serum (Hyclone US),
100U/ml penicillin and 100μg/ml streptomycin. The cells were put
in a humid atmosphere containing 5% CO2, with medium changed
first 5 days and every 3 days thereafter.
3. Co-culture of meniscal cells and bMSC
After 10–14 days of primary culture, when the proliferating
colonies had nearly reached confluence, the adherent cells were
harvested with 0.25% trypsin-ethylenediaminetetraacetic acid.
The cells were prepared to co-culture without contact. All
co-cultures were conducted in 6-well plates and using
1.0 um
PET transparent
hanging cell culture inserts
(Millipore,USA). MSCs were seeded on 6-wells plates, while
meniscal cells were seeded on the upper surface of the membrane
of the cell culture inserts.
1x105 cells were seeded into each well or membrane of
the tissue insert.
Co-cultured cells were maintained for 7 days in DMEM/F12 with
10% fetal bovine serum at 37°C and 5% CO2 in a humidified
atmosphere with medium being exchanged every 3 days. As
controlling, MSCs and meniscal cells were seed into 6-well
plates separately
1x105 cells
per well in 6-wells plates.
4.
Immunohistochemistry of type I Collagen and type III expression
Expression of type I collagen and type III collagen was assessed
by immunohistochemistry(IHC), using monoclonal anti- collagen
type I(Sigma, USA) and collagen III(
Calbiochem USA).
All incubations were performed in a humified chamber. All
adherent cells were fixed by acetone.the samples of cells were
incubated overnight at 4°C with anti- collagen type I and
collagen III at a dilution of 1:200. The Collagen I reaction was
visualized by using EnVision+ Dual Link DAB (zhongshanjinqiao,Beijing,
China) according to manufacturers’ list with hematoxylin
counterstaining.
5. Real-time PCR analysis of gene expression
Cells were rinsed in PBS and lysed in TRIzol (Invitrogen). Total
RNA was then extracted following manufacturers constructions.
Briefly, chloroform was added to each sample and sample tubes
centrifuged to enable phase separation. RNA was precipitated by
addition of isopropanol to the aqueous phase, followed by
centrifugation. Precipitated RNA pellets were washed in 75%
ethanol and then resuspended in distilled RNAse-free water. cDNA
was prepared from RNA using
Revert Aid™ Frist Strand
cDNA Synthesis Kit
(MBI). RNA (1<μg) was mixed with random prime hexamers (200ng)
then incubated at 70°C for 5 minutes. Tubes were cooled on ice,
then 5x first strand buffer,
10mM
dNTP mix 2ul,
20U
Ribonuclease Inhibitor (recombinant)
and 200U
RevertAid™ M-MuLV
Reverse Transcriptase
were added, giving a final volume of 20μl. Samples were then
incubated at 20°C for 10 min, 42°C for 60 minutes and finally
heated to 70°C for 10 minutes.
Gene expression was analyzed by real-time PCR using an
FTC2000
(Funglyn
Canada).
Housekeeping genes was GAPDH. Primers for collagen type I, II,
III and aggrecan were designed using the Primer Premier 5.0
software(Table 1). Reactions were carried out in duplicate in
96-well plates in a final volume of 30μl. For GAPDH , the
reaction mix contained
10×buffer(Mg2+ free), 3 µl MgCl2(25mM), 0.36
µl dNTP(25mM), 1 µl, upstream primer (10 µM), 1 µl downstream
primer(10 µM), primer(10 µM),3U Taq, ,ddH2O 18.74 µl,
5 µl cDNA.
The PCR reaction consisted of an initial enzyme activation step
at 94°C for 2 minutes, followed by 40 cycles of 94°C for 20
seconds, 55°C for 30 seconds and 60°C for 40 seconds. A cycle
threshold value (Ct) value was obtained for each sample and
duplicate sample values were averaged. The 2-ΔΔCt method was
then used to calculate relative expression of each target gene.
|
Primer |
Primer sequences
Forward and Reverse
|
Amplicon size |
GENE Bank
Accession number |
|
GAPDH |
F:AACCACGAGAAGTATGACAACT
R:CGTGCACCGTGGTCATGAG |
133 |
L38480 |
|
AGGRECAN |
F:TACCAGGACAAGGTCTCGCT
R:GGAACACAACACCTTTCACCA |
169 |
L23961 |
|
Type I collagen |
F:
GATGGCTTCCAGTTCGAGTA
R:
GCCACGCTGTTCTTGCAGT |
120 |
AY633663 |
|
Type II collagen |
F:CTTCCACTTCAGCTATGGAG
R: GCCACGCTGTTCTTGCAGT |
126 |
D83228 |
|
Type III collagen |
F:
CATTGGCCCTGTTTGCTTTT
R:
CTTCCTGAAGCCCCAGCAGA |
110 |
S83371 |
Table 1. Real-time PCR primer details. Accession number given
for primers designed using Primer Premier 5.0 software.
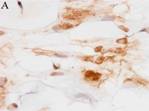 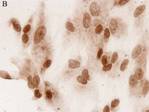 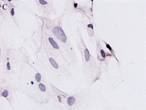
Fig 1.type I collagen protein was found in MSCs cells which had
co-cultured with meniscal cells without contact(A 400X) and
meniscal cell(B 400X) and type I collagen protein wasn’t found
in controlled MSCs.(C 400X)
 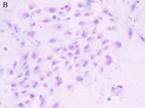 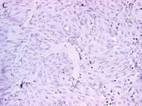
Fig 2: collagen type III protein was not found in MSCs
cocultured meniscal cell(A 200X), meniscal cell(B 200X) and
controlled MCSs(C 100X)
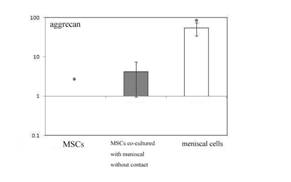
Fig 3 expression of aggrecan mRNA by controlled MSCs , MSCs
following co-culture without contact and meniscal cell following
7 days in culture.
. * = statistical significance (P<0.05) from MSCs following
co-culture without contact
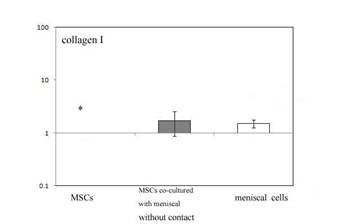
Fig 4 expression of type I collagen mRNA by controlled MSCs ,
MSCs following co-culture without contact and meniscal cell
following 7 days in culture.
. * = statistical significance (P<0.05) from MSCs following
co-culture without contact
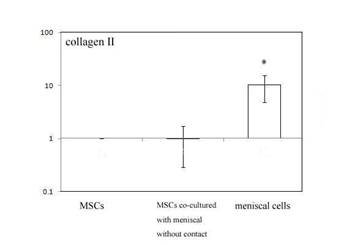
Fig 5 expression of type II collagen mRNA by controlled MSCs ,
MSCs following co-culture without contact and meniscal cell
following 7 days in culture.
. * = statistical significance (P<0.05) from MSCs following
co-culture without contact
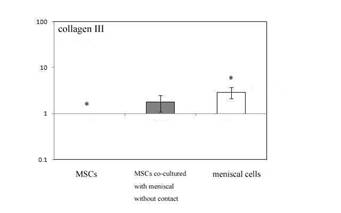
Fig 6 expression of type III collagen mRNA by controlled MSCs ,
MSCs following co-culture without contact and meniscal cell
following 7 days in culture.
. * = statistical significance (P<0.05) from MSCs following
co-culture without contact
Statistical analysis
Statistical significance was determined using Pair Wise Fixed
Reallocation Randomization Test with calculation SPSS 13.00
software, where P <0.05 was considered significant.
Results :
Immunohistochemistry
Collagen type I protein was found in MSCs cells which had
co-cultured with meniscal cells without contact (fig 1 A). While
in controlled samples, the expression of collagen type I protein
was found in meniscal cells (Fig 1B).In the other hand, the
expression of collagen type I protein wasn’t found in MSCs solo
cultured as control.(Fig 1C).
The collagen type III protein wasn’t found in MSCs cells which
had co-cultured with meniscal cells without contact(fig 2 A),
meniscal cells(fig 2 B) and MSCs solo cultured as control(fig 2
C) .
Gene expression following meniscal cells and MSC co-culture
without contact
The mRNA expression of aggrecan, type I collagen, type II
collage and type III collagen were assessed by real-time
quantitative RT-PCR (Fig. 3). Group I was the MSCs solo
cultured, Group II was the MSCs co-cultured with meniscal
without contact, Group III was meniscal cells as control.
The resulting data were expressed as a ratio of group I. As
shown in (Fig. 3), the expression of Aggrecan mRNA of Group II
was significantly (P<0.05) increased than Group I and also
significantly(P< 0.05) lower express than that in Group III
after 7 days culture. In addition, the ratios of Aggrecan for
group II and group III were 4.15 ± 3.19 and 54.14 ± 19.8,
respectively. The expression of type I collagen in three group
was show in (Fig. 4). the type I collagen mRNA of group II was
significantly (P< 0.05)increased than Group I,and
then slightly lower than that of the Group III(P>0.05) after 7
days culture. The ratios of type I collagen of group one for
group II and group III were 2.91 ± 0.80 and 1.78 ± 0.69. In
(Fig.5), the type II collagen mRNA of group II was higher but
not significantly (P>0.05) and significantly lower than that of
Group III (P < 0.05) after 7 days culture. The ratios of type II
collagen for group II and group III were 0.99 ± 0.70 and 10.27 ±
5.44.In addition, the type III collagen mRNA of group II was
increased than that of group I but not significantly (P<0.05)
and then as the same as the group III(P<0.05) after 7 days
culture. The ratios of type III collagen for group II and group
III were 1.72 ±0.84 and 1.52 ± 0.25. (Fig.6)
Discussion :
Tissue engineering or cell-based therapies for repair of the
require a large number of cells (e.g. 25x106 -37 x106
cells/ml (14,15,16)]) with an fibrocarilage-like
phenotype that can be implanted into the meniscal scaffold to
produce a new matrix. It can be expected that only cells from
the torn inner parts of meniscus will be available. The number
of these cells will be small and the quality may be compromised
by the trauma. So far no culture experiments have been described
using this cell source and it can be doubted if these cells will
proliferate and differentiate into the desired phenotype. While
autologous MSCs would be the ideal choice, studies have shown
that MSCs lack HLA class II receptors (17) and that
human recipients receiving MSCs from sibling donors do not
exhibit an immunogenic response (18)suggesting that
allogeneic MSCs could be used. However, only using MSCs has some
limitations when combine with some scaffolds. Port et al.
reported on meniscal repair supplemented with exogenous fibrin
clot and BM-MSCs in a goat model, but the addition of BM-MSCs in
conjunction with the fibrin clot did not enhance meniscal
healing. (19) MSCs with hyaluronan-ester and gelatin
scaffold have been used to treat fibrocartilage defects in a
rabbit model, but the ‘meniscus’ didn’t completely restore the
surface area and the tissue quality of normal meniscus.
(20)
The main problem for these strategies is the generation of a
usable cell population.
Until to now, there are no methodology that can be employed to
induce and maintain a differentiated phenotype in MSCs,
involving addition of growth factors or culture in a
three-dimensional environment. The current study aimed to
elucidate the effects co-culture had on the differentiation
state of both meniscal cells and MSCs.
Our study suggested that co-culture without contact show some
significantly change in matrix gene expression and protein
secretion. This was similar with other studies using different
cell types co-cultured with MSCs where no cell-cell contact has
been shown to have some effect. But some studies demonstrate the
different result that there was no effect when using co-culture
without contact. (21) Our results may therefore be
due to the specific cell type i.e. the meniscal cell, use for
co-culture.
Our study shows that after co-culture has more collagen I
protein mRNA expression and have secretion of collagen I protein
comparing to controlled MSCs. This is important as collagen I
protein is a mainly component of meniscal tissue (22)
and has been shown to be expressed by meniscal cells in our
study. Moreover, collagen I protein mRNA expression of MSCs was
only slightly lower than that of controlled meniscal cell. The
co-culture have been shown to stimulate aggrecan expression in
MSCs and this could count for fibro-cartilaginous
lineage differentiation.
There was, however, no increase in type II collagen in MSCs
following co-culture with meniscal cell and type II collagen was
still less than that of controlled meniscal cell. the same
result was reported in previous study (22).
While type III collagen was only minor (22
-24 ),
yet still significant changes in expression, results suggest
that type III collagen was expressed at same levels by both
meniscal cells and MSCs following co-culture. Base our study,
the MSCs has some potential to differentiate to
fibro-cartilaginous
lineage because of increasing expression of type I collagen,
aggrecan and type III collagen.
This study had several limitations. First, only the co-culture
method without contact was applied in our study. Second, there
was only one cell ratio (1:1) when apply cell culture. In
further research, cell-to-cell culture method and more cell
ratio cultures should be used.
In conclusion we have shown that co-culture of meniscal cells
and MSCs causes MSCs differentiation to a fibrocartilage-like
phenotype following co-culture without contact. Whereas the cell
was not the same as the fibrochondrocyte, the MSCs have the
potential to differentiate to fibrocartilage phenotype. However,
the current strategy could be an alternative approach for
supplying the seed cell of tissue engineering of meniscus.
Reference:
-
Poehling GG, Ruch DS, Chabon SJ. The landscape of meniscal
injuries. Clin Sports Med 1990;9:539–549
-
Allen PR, Denham RA, Swan AV: Late degenerative changes after
meniscectomy: Factors affecting the knee after operation. J
Bone Joint Surg 1984;66B:666–671.
-
Mckinley TO, English DK, Bay BK. Trabecular bone strain
changes resulting from partial and complete meniscectomy. Clin
Orthop, 2003; 407: 259~267.
-
Andersson-Molina H, Karlsson H, Rockborn P. Arthroscopic
partial and total meniscectomy: a long- term follow-up study
with matched controls.arthroscopy.2003;18:183–189
-
Arnoczky S, Warren R, Spivak J. Meniscal repair using an
exogenous fibrin clot. An experimental study in dogs. J Bone
Joint Surg 1988;70:1209–17.
-
William D. Bugbee Fresh Osteochondral Grafts for the Knee
Techniques in Knee Surgery 2004;3(2):68–76.
-
Oleg Safir, David Backstein, Paul Zalzal et,al Massive
Osteochondral Allografts in the Management of Nontumoral
Conditions Around the Knee Techniques in Knee Surgery
2005;4(2):89–99
-
Arnoczky SP. Buiding a meniscus. Clin Orthop 1999;367(suppl)
:s244-532
-
Gwendolyn M. Hoben and Kyriacos A. Athanasiou. Meniscal
Repair With Fibrocartilage Engineering Sports Med Arthrosc
Rev 2006;14(3):129-137.
-
Christian Jorgensen, Jan Gordeladze and Danielle Noel Tissue
engineering through autologous mesenchymal stem cells Current
Opinion in Biotechnology 2004; 15:406–410
-
Pereira RF, Halford KW, O’Hara MD et, al.
Cultured adherent cells from marrow can serve as long-lasting
precursor cells for bone, cartilage, and lung in irradiated
mice. Proc Natl Acad Sci USA 1995; 92:4857-4861.
-
Webber, R. J., Harris, M., and Hough, A. J.: Cell culture of
rabbit meniscal fibrochondrocytes: Proliferative and synthetic
response to growth factors and ascorbate. J. Orthop. Res.
1985;3:36-42.
-
Webber RJ. In vitro culture of meniscal tissue. Clin Orthop
1990,252:114–20
-
Takahashi K,Hashimoto S,Kubo T, et a.l .HyaIuronan suppression nitric oxide production in
meniscus and synovium of rabbit osteoarthritis modal .J
Orthop Res 2001;19(3):500—3
-
Spindler KP, Mayes CE, Miller RR, et al. Regional mitogenic
response of the meniscus to platelet-derived growth factor (PDGF-AB).
J Orthop Res 1995;13:201–7
-
Lietman SA, Hobbs W, Inoue N, et al.
Effects of selected growth factors on porcine meniscus in
chemically defined medium. Orthopedics. 2003;26:799–803
-
Le Blanc K, Tammik C, Rosendahl K et al. HLA expression and
immunologicproperties of differentiated and undifferentiated
mesenchymal stem cells Exp.Hematol. 2003;31:890-896
-
Horwitz EM, Gordon PL, Koo WK et al. Isolated allogeneic bone
marrow-derived mesenchymal cells engraft and stimulate growth
in children with osteogenesis imperfecta: Implications for
cell therapy of bone. Proc.Natl.Acad.Sci.U.S.A
2002;99:8932-8937
-
Port J, Jackson DW, Lee TQ, Simon TM. Meniscal repair
supplemented with exogenous fibrin clot and autogenous
cultured marrow cells in the goat model. Am J Sports Med
1996;24:547– 555.
-
Angele P, Johnstone B, Kujat R, Zellner J, Nerlich M, Goldberg
V, et al. Stem cell based tissue engineering for meniscus
repair. J Biomed Mater Res A 2008;85:445–55.
-
Stephen M. Richardson, Rachael V. Walker, Siân Parker, et,al
Intervertebral Disc Cell Mediated Mesenchymal Stem Cell
Differentiation Stem Cells 2005;(0205):1-43
-
Christian Jorgensen, Jan Gordeladze and Danielle Noel Tissue
engineering through autologous mesenchymal stem cells. Current
Opinion in Biotechnology 2004;15:406–410.
-
McDevitt CA, Webber RJ. The ultrastructure and biochemistry of
meniscal cartilage. Clin Orthop 1990; 252:8-18.
-
Eyre DR, Wu JJ. Collagen of fibrocartilage: a distinctive
molecular phenotype in bovine meniscus. FEBS Lett
1983;158:265-70
|












