|
Abstract:
J.Orthopaedics 2010;7(2)e8
Keywords:
Introduction:
There is a relatively
high prevalence of calcaneus, tibial plateau, tibial pilon, and
distal radius fractures in modern society with a growing
economic cost related both to the expense of treatment as well
as ongoing disability 1-4. Calcaneus, tibial plateau
and tibial pilon fractures are especially known for their high
rates of wound complications 5-10. Wound
complications and disturbed fracture healing are further
complicated by medical comorbidities that are increasingly
common, including obesity, diabetes mellitus and smoking
11-19. The significance of these
complications is heightened by the fact that these fractures
tend to occur in younger patients, and our society continues to
live longer 1, 20-21.
In order to reduce fracture non-unions and wound complications,
new minimally invasive techniques for fracture management
continue to be developed. One such technique that has had great
success is balloon kyphoplasty (Kyphon / Medtronic, Inc,
Sunnyvale, CA) 22-24, which until now has been
relegated to the treatment of compression fractures of the
spine. Kyphoplasty is generally performed using an inflatable
balloon, which is inserted from a posterior transpedicular or
extrapedicular approach in the affected vertebral body. The
height of the compressed vertebra is restored by insufflation of
the balloon with carefully-regulated pressurized liquid. This
device is known as the Inflatable Bone Tamp (IBT, Kyphon /
Medtronic, Inc, Sunnyvale, CA). The IBT is designed to
“compress cancellous bone and/or move cortical bone as it
inflates”25. It is comprised of three biocompatible
parts: a proximal luer fitting, a central catheter, and a distal
inflatable tip with radiopaque markers. Inflation of the
balloon is achieved by using an external inflation syringe
filled with radiopaque dye (i.e. OmnipaqueTM)
connected to the proximal luer-lock connection. This inflation
syringe measures the volume (cc) and the pressure (psi) of the
inflation. These characteristics allow the device to be used in
any bone simply as a conventional bone tamp or as a percutaneous
bone tamp with fluoroscopic guidance. The inflatable bone tamp
is FDA-approved for use “as conventional bone tamps for the
reduction of fractures and/or creation of a void in cancellous
bone in the spine, hand, tibia, radius and calcaneus” 26.
Pre-market testing in cadaveric fractured tibial plateaus and
unfractured vertebrae has been performed, demonstrating that the
IBTs can reduce cortical fractures and create voids in
cancellous bone in the same manner as conventional bone tamps.
Safety testing also demonstrated no increase in risk over
conventional bone tamps 25. Interestingly, despite
years of successful use in the spine, the IBT has never been
formally developed by surgeons as a reduction tool in extremity
fractures, despite having FDA approval for such applications.
The lead author (henceforth referred to as the author) has
explored and developed a novel technique for reduction of
peri-articular extremity fractures using an IBT and a
fast-setting calcium phosphate cement. The placement of
fast-setting calcium phosphate cements into fresh orthopaedic
fracture sites has been proven beneficial in filling bone voids,
such as those that remain after reduction of impacted articular
fractures 27-36. In
practice, the reduction of impacted articular fractures as well
as bone-grafting of residual metaphyseal defects can be
difficult, especially when using minimally invasive methods.
The author has found that the technique of kyphoplasty
balloon-assisted reduction of impacted lower extremity articular
fractures to be a reproducibly successful approach that is
adaptable to many fractures. This minimally invasive technique
includes the reduction of articular surfaces using a
percutaneous balloon and fluoroscopic control, followed by
insertion of an appropriate amount of fast-setting calcium
phosphate cement into a well developed and positioned void, and
finally placement of fracture fixation hardware as needed.
Surgical Technique
Since early 2009, the author has successfully used the KyphX
Xpander® Inflatable Bone Tamp (IBT, Kyphon, Sunnyvale, CA)
balloon for extremity fracture reduction in numerous cases. The
author is experienced using the KyphX Xpander® IBT (Kyphon /
Medtronic, Inc, Sunnyvale, CA), therefore the technique
described herein will refer to this device. However, this
technique is not limited to this particular IBT and other
inflatable devices could potentially be used. This technique
was used in a variety of fractures, including distal radius,
tibial plateau, tibial pilon and calcaneus. The general
technique is described in this report, and two specific case
examples are presented to illustrate the technique used.
Finally, we will briefly present technical tips for use in two
other fracture locations.
Step 1: Pre-operative planning
Radiographs of the affected extremity are essential for
preliminary assessment of the fracture. Depending on the extent
of the fracture (e.g. comminution, cortical shell integrity,
etc.) and the condition of the soft tissues, a patient may need
to be placed into an external fixator prior to definitive
fracture reduction and fixation. A computed tomography (CT)
scan is recommended in cases with intra-articular involvement or
comminution. To best plan for the procedure, all images should
be carefully examined to assess deformity, number and location
of bone fragments, location and amount of articular depression,
and other areas of concern to determine the size of balloon and
type of reduction equipment needed. Prior to the case, it is
important to take inventory of all the equipment necessary for
the case. The basic equipment used to carry out balloon
reduction and minimally invasive
fixation (we have coined the term BRAMIF) is the KyphX®
Osteo Introducer® system, an additional single Kyphon Balloon®,
bone filler (a fast-setting calcium phosphate cement), OmnipaqueTM,
C-arm fluoroscopy, and typical reduction tools such as forceps,
k-wires, plates, plate-holding clamps, power drill, etc.
Additionally, all radiographs and CT scans should be available
in the operating room for reference. The approach for minimal
soft-tissue damage and internal fixation should be
pre-determined. The desired internal fixation hardware should
be available.
Step 2: Provisional Reduction & Fixation
The initial surgical tactic should focus on provisional fracture
reduction and fixation. First, a small incision is made and the
fracture line is exposed as needed. The primary fracture
fragments should be provisionally approximated using k-wires,
clamps, reduction forceps, and/or the plate. In the case of
intra-articular fractures and/or joint line depression, it is
recommended that stable reduction and fixation of all fracture
lines on the diaphyseal side of the defect or desired balloon
location be carried out first. This ensures the balloon, once
inflated, will take the path of least resistance and act upon
the desired area of impacted articular surface and cancellous
bone, rather than serve to displace another portion of the
fracture. Also, hardware (such as k-wires or screws) may be
applied around the area of articular depression to “frame” the
impacted area, which helps to ensure that expansion of the
balloon occurs in the desired direction to affect reduction of
the articular fragments. This allows the balloon to reduce the
depressed portion of the fracture appropriately without “escape”
of the balloon or calcium phosphate when inserted.
Step 3: Balloon Preparation and Insertion
The desired location of the balloon is determined during
pre-operative planning, and the balloon is inserted
percutaneously into this area once provisional fixation (and
reduction if necessary, see Step 2) is achieved. The balloon
should be positioned so that the long axis of the balloon is
parallel to the joint line and just beneath the impacted defect
in the bone, but with a shelf of bone between the balloon and
any articular surfaces. Using fluoroscopic guidance, the KyphX®
Osteo Introducer® system is used to position the uninflated
balloon in the desired location. The introducer system includes
a cannula, hand drill, and spade- and diamond-tip trocars.
First, a trocar and cannula is placed on the outer cortex of the
bone, and a mallet is used to impact the trocar through the
cortex and into the cancellous bone. In the event that this
access is difficult, a power drill may be used to facilitate
entry through cortical bone. It is important that this be done
gently in order to not cause further fracture, especially in
osteoporotic bone.
Once access is gained to cancellous bone, the trocar is removed
while leaving the cannula in place, and a hand drill is used to
make a path for the balloon through the cancellous bone. The
depth of entry should be determined by using the depth markers
on the hand drill and the appropriate balloon size can be
determined (e.g. 10mm, 15mm, 20mm). Therefore, it is beneficial
to have several balloon sizes available. The appropriate
balloon/inflation syringe assembly is filled with contrast
medium (i.e. OmnipaqueTM) and is readied for
insertion. It is important to make sure that the area within
the bone where the balloon will be deployed is clear of any
sharp bone fragments, screws or k-wires that might damage the
balloon or cause it to break upon inflation. The balloon is
then inserted through the KyphX® Osteo Introducer® system until
the two radiopaque markers (representing both ends of the
balloon) are seen outside the cannula and are completely within
the bone in the desired location.
Step 4: Balloon Expansion and Fracture Reduction
Before inflating the balloon, be sure to check its position with
fluoroscopy. Two radiopaque markers, representing both ends of
the balloon, should be seen outside the cannula, and beneath the
impacted bone fragment. Once it is confirmed that the balloon
is properly placed (parallel to the joint line, beneath the
impacted bone), the knob on the inflation syringe is slowly
turned to inflate the balloon with the contrast. A fluoroscopic
image should be taken with each additional 0.5 to 1 cc of
balloon inflation to ensure the balloon has not moved or
deformed, and to assure that fracture reduction and bone void
formation is occurring as desired. Continue inflating the
balloon without exceeding the volume or pressure recommendations
determined by the manufacturer. The volume of expansion should
be recorded in order to estimate the volume of bone void filler
that will be needed. The balloon may be deflated once the
desired fracture reduction, cancellous bone impaction and bone
void formation has been achieved. Upon deflation of the
balloon, if it is apparent that further reduction is needed, the
balloon may be repositioned and reinflated by repeating the
above steps, all while obtaining appropriate fluoroscopic images
and recording the total volume of expansion.
In the case of a large bone defect or void, the “double
stacking” technique may be used (most often necessary in the
calcaneus). This technique requires the availability and use of
two separate balloons. This technique is used when the defect
in the bone is too large for one balloon to fill. When this is
the case, repositioning the original balloon closer to the
impacted bone and attempting reinflation for further reduction
may not be successful because the balloon may simply expand back
into the original void and not affect any further fracture
reduction. However, with the additional deployment of a second
balloon along with the still-inflated first balloon, the defect
can be fully filled and the fracture properly reduced. To use
the stacked balloon technique, first deploy and inflate one
balloon according to the above instructions and leave it in
place. Next, carefully introduce a second cannula parallel to
the first balloon and closer to the still-displaced bone
segment, while being careful not to damage the first balloon
with any of the instrumentation. Finally, inflate the second
balloon as previously instructed until the fracture is properly
reduced and a void is created. As before, the total volume of
both balloons should be noted to estimate the amount of bone
void filler that will be needed to graft the defect. At this
point, the balloons should have compacted the cancellous bone
supporting the comminuted fragments and created a tightly sealed
void for the bone filler to be placed.
Step 5: Bone Filler Preparation and Insertion
The appropriate amount of bone filler is chosen according to the
volume measurements obtained from the inflated balloon(s). The
authors have used a drillable, fast-setting calcium phosphate
cement, such as HydrosetTM (Stryker; Kalamazoo, MI)
37-38. The luer lock of the HydrosetTM
kit works directly with the Kyphon® bone filler device,
facilitating introduction of the bone filler. Although the
remainder of this technique is described for this particular
product, another fast-setting calcium phosphate substitute (e.g.
Norian SRS®) may be chosen. Some surgeons may choose to use the
more traditional cancellous autograft or allograft. Please note
that if the surgeon chooses to use another bone-graft
substitute, an alternative method to deliver the bone void
filler into the defect will have to be used. For example, a
transfer syringe may be needed if the luer lock of the delivery
system for the desired material does not match that of the
Kyphon® bone filler device.
The calcium phosphate is prepared according to manufacturer’s
instructions, paying particular attention to time constraints,
and making sure that enough volume of filler is available. Once
mixed, the syringe is assembled and filled with the material. A
Kyphon® bone filler device is attached to the luer lock of the
syringe and filled with the calcium phosphate cement. Several
bone filler devices are filled with the calcium phosphate cement
until all available material is used.
Each fully-loaded bone filler device with a pusher stylet should
be handed to the surgeon as soon as it is filled. It is
important to emphasize that one must work quickly during this
step so that the calcium phosphate cement does not harden before
it can be injected into the bone. Each filled bone filler
device is inserted into the cannula to a distant point in the
void and its position confirmed with fluoroscopy. Insert the
pusher into the back of the bone filler device and slowly inject
the calcium phosphate while gradually pulling back on the bone
filler device as needed to “backfill” the entire space. Several
bone filler devices may be required to fill the entire void. Be
sure the scrub technician is well-instructed prior to starting
this process, since time is of the essence and efficiency is
important during the bone filler exchange process. Once the
void is filled with calcium phosphate, the bone filler device
and cannula are removed. Please note that this step in the
process is very similar to that of kyphoplasty. Therefore, if
the surgeon does not perform kyphoplasty as a part of their
practice, they should gain assistance from their Kyphon® or
alternative IBT representative.
Step 6: Internal Fixation
The final step is to complete the internal fixation of the
fracture with the desired hardware, as determined during the
pre-operative planning stage. Please refer to the
manufacturer’s instructions on recommendations in regards to
drilling or placing screws into the bone filler.
Illustrative Cases
Example Case #1: Distal Radius Fracture
A sixty-seven year-old female presented to the emergency room
after slipping on black ice, injuring her left wrist. She had
obvious deformity of her wrist, and radiographs revealed a
dorsally-displaced left distal radius fracture (Figure 1A). The
patient was given moderate sedation and her wrist was
manipulated and reduced by closed means. Her wrist was initially
splinted and post-reduction films were obtained, which revealed
the distal carpus continued to be displaced dorsally (Figure
1B). At that time, she was counseled on the options of closed
versus open treatment and the patient selected open treatment.
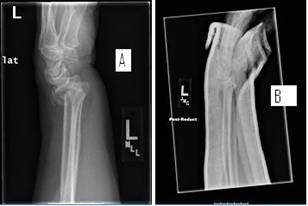
Figure 1: (A) Initial presentation showing dorsal displacement
of the distal radius and (B) a lateral post-reduction
radiograph.
The patient was taken to the operating room. Her arm was placed
on a radiolucent table and was sterilely prepped and draped. A
small volar incision was made and dissection was carried out
down to the bone to expose the fracture line. A volar, locking
distal radius plate was chosen. Other specific steps of this
case are described through the following series of images and
descriptions (Figures 2-4).
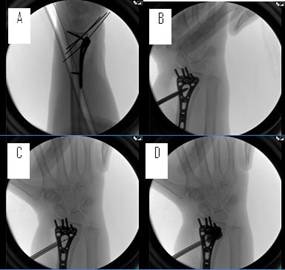
Figure 2: (A) Provisional reduction and fixation was carried out
by using a volar, locking distal radius plate, k-wires and
non-locking screws framing the fracture. (B) Using the KyphX®
introducer system, the port for the balloon was created using a
radial approach with a trocar and a path was created with a hand
drill through the cannula. (C) The balloon was inserted through
the cannula until the two radiopaque markers were seen outside
the cannula and in the desired location while remaining
completely within the bone. (D) The balloon was then inflated as
to not exceed the pressures indicated by the manufacturer. The
inflation of the balloon better reduced the joint line, moved
the distal fragment to the plate and impacted all the
surrounding cancellous bone in order to create a void that would
not allow escape of the bone filler through the intra-articular
split. The volume of the balloon was recorded in order to
prepare the proper amount of bone filler needed in the next
step. Finally, the balloon was deflated and removed while
leaving the cannula in place.
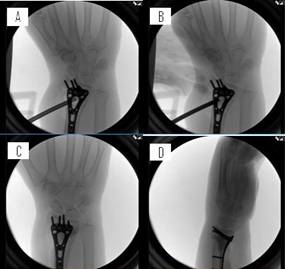
Figure 3: (A-B) The void created by inflating the balloon is
seen with the cannula in place. At this point, after taking note
of the balloon inflation volume, 3cc of calcium phosphate cement
was mixed and prepared for insertion. These steps were all done
in a rapid, efficient manner as to not allow the fast-setting
calcium phosphate to harden prior to insertion. The
syringe was attached to the Kyphon® bone filler device and two
bone filler devices were filled with calcium phosphate (1.5cc
each). The authors began filling the void by inserting the bone
filler device to the furthest point and “backfilling” the void
while steadily pulling back on the bone filler device as
needed. The void was completely filled with calcium phosphate
while experiencing no leaks and minimal waste as seen from both
the (C) AP and (D) lateral views.
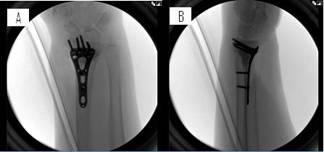
Figure 4: Internal fixation was completed with application of
various non-locking and locking screws in the volar distal
radius plate, shown by both (A) AP and (B) lateral views.
Example Case #2: Tibial Plateau Fracture
A fifty-six year old female involved in a motor vehicle accident
complains of right knee pain. Initial radiographs reveal a
displaced fracture of the right lateral tibial plateau and right
proximal fibula fracture (Figure 5A-B). Due to the poor
condition of the soft tissues, the patient was placed into a
knee-spanning external fixator and a CT scan was obtained to
further evaluate the extent of the tibial plateau fracture
(Figure 5C-D).
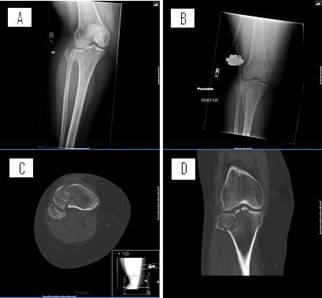
Figure 5: (A) Initial radiograph at presentation. (B) Radiograph
after application of external fixator. (C) Axial and (D) coronal
CT scan sections following external fixation. The CT scan was
obtained to analyze the severity of comminution and articular
involvement.
The patient was discharged for two weeks and returned at that
time for definitive fixation of her tibial plateau fracture.
She was placed on a radiolucent surgical table and was sterilely
prepped and draped. A small vertical anterolateral incision was
made and dissection was carried out down to the bone to expose
the vertical-split fracture and the knee joint line. An injury
was noted to the lateral meniscus, so stay sutures were placed
in the meniscus until it could be repaired at the end of
surgery. A large C-arm was used throughout the procedure to
check the reduction and placement of hardware. Next, the
appropriate proximal tibial plate was chosen and the other
specific steps of this case are described though the following
series of images and captions (Figures 6-8).
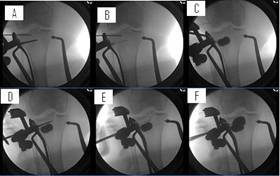
Figure 6: Provisional reduction and fixation was carried out by
placing a lateral proximal tibia plate in position and holding
this position with large reduction forceps and k-wires. Using
the KyphX® introducer system, a port for the balloon was created
using a lateral approach and gently tapping a trocar through the
cortical bone. (A) A path was then created with a hand drill
through the cannula. (B) The balloon was inserted through the
cannula until the two radiopaque markers were seen outside the
cannula. The proximal k-wire holding the provisional reduction
was removed so the balloon could act upon the joint line. (C)
The balloon was then inflated as to not exceed the pressures or
volume indicated by the manufacturer. The balloon reached
maximum capacity while remaining low in pressure with incomplete
reduction, therefore indicating a void too large for one balloon
(see discussion regarding balloon pressure readings). We then
carried out the “double stacking” technique. We left the first
balloon inflated inside the bone and introduced a second balloon
just above the first, using the same technique as for the first
balloon. An additional trocar was used to make a port through
the cortical bone and (D) the hand drill was carefully
introduced using fluoroscopy guidance as to not damage the first
balloon. Once the appropriate path was made for the balloon,
(E) the balloon was inserted so the two radiopaque markers were
seen outside the cannula while remaining within the bone. (F)
The balloon was inflated until the void was filled and the joint
line reduced as to not exceed the volume and pressure indicated
by the manufacturer. The volume of both balloons was recorded
in order to prepare the proper amount of bone filler needed in
the next step. Finally, the balloons were deflated and removed
while leaving the two cannulas in place.
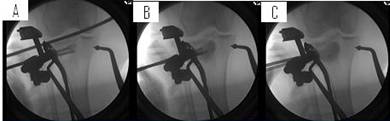
Figure 7: At this point, after taking note of the balloon
inflation volume, the calcium phosphate was mixed and prepared
for insertion as previously described in the general technique.
(A) We began filling the void by inserting the bone filler
device to the furthest point and (B) “backfilling” the void
while steadily pulling back on the bone filler device as needed.
(C) The void was completely filled with calcium phosphate while
experiencing no leaks and minimal waste.
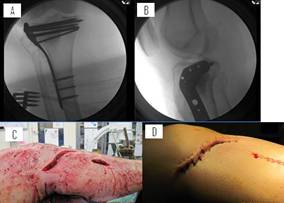
Figure 8: Finally, the tibial plateau was internally fixated
using a lateral proximal tibia plate and screws shown in an (A)
AP and (B) lateral view, while also showing the minimally
invasive incisions used to carry out this technique. The
incisions are shown (C) after hardware implantation and prior to
closure and (D) after closure.
Case #3: Calcaneus Fracture
For specific tips on treatment of calcaneus fractures see Figure
9.
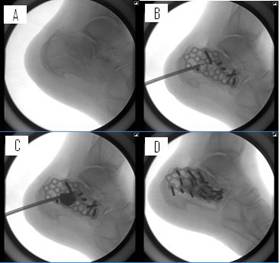
Figure 9: These figures illustrate the general use of BRAMIF in
the calcaneus. (A) Pre-surgical image. The general technique
follows that of the previous cases, but (B) this image shows the
provisional fixation while, most importantly, highlighting the
balloon position through a posterior calcaneal approach, which
is a notable difference from the usual position parallel to the
joint line. (C) The balloon was inflated to raise the joint
line. Also, in the calcaneus, the bone void is typically large
enough that complete reduction requires use of the “double
stacking” technique. (D) The final image shows the void filled
with the appropriate volume of calcium phosphate and internally
fixated with a calcaneal plate and screws. Alternatively,
completely percutaneous internal fixation may be used in some
cases.
Case #4: Tibial Pilon Fracture
This tibial pilon fracture is a good example of the “framing”
technique, which involves application of hardware around the
area of articular depression and the cancellous bone void that
will be present after reduction (Figure 10). This best reduces
the depressed portion of the fracture without allowing “escape”
of the calcium phosphate.
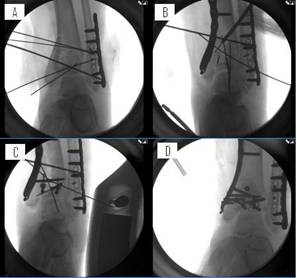
Figure 10: (A) Shows an initial attempt at provisional fixation,
which will provide no balloon capture. The fracture is too
unstable, so if the balloon were inflated, the fractured
fragments would spread in all directions, while leaving the
joint line depressed. Instead, (B) the depressed joint line was
“framed” with k-wires laterally, distally and proximally, and a
plate medially. As an additional tip, the k-wires can have
cannulated screws placed over them should the surgeon desire a
more sturdy “frame”. A balloon was then placed above the area
of articular depression and inflated to reduce the impacted
articular segment and compact the underlying cancellous bone.
Once a bone void was created and the balloon was removed, a
large posterior fragment was fixed with screws and a distal
screw was placed in the plate. (C) These screws provided a
medial, lateral and superior frame around the previously created
bone void to assist in preventing escape of the bone filler and
while stabilizing the bone surrounding the void. Calcium
phosphate cement was then inserted using the Kyphon® bone filler
devices. (D) The void was completely filled without escape of
calcium phosphate and the distal tibia was internally fixed with
the plate and screws.
Discussion :
While the application of an inflatable balloon tamp (IBT) as an
internal bone reduction device is not a new concept, the
application of this balloon reduction and minimally invasive
fixation, BRAMIF, method to
periarticular fractures of the extremities is a novelty. The
IBT, particularly the Kyphon ® balloon, is best known for the
reduction of vertebral body fractures; a technique known as
balloon kyphoplasty. During kyphoplasty, the balloon is
percutaneously inserted into the vertebral body under
fluoroscopic control, and then inflated to reduce the fracture
and impact cancellous bone 22-24. Next, bone cement
(i.e. polymethylmethacralate, PMMA) is inserted into the
residual bone void in the vertebral body in order to support the
fracture reduction and maintain the vertebral body height. By
compacting cancellous bone, the balloon also reduces the
likelihood of PMMA from escaping outside the vertebral body
22, 39-41.
This technique has been used in over 600,000 spinal fractures to
date (electronic mail: Denise Moore,
Communications, Kyphon Products,
Sunnyvale, CA). Several studies, including a prospective
randomized control trial 22, have reported improved
quality of life, improved disability measures and a reduction of
back pain using this technique 22-23, 39. Therefore,
the authors believe it would be beneficial to achieve the same
benefits of percutaneous fracture reduction in the extremities,
especially fractures associated with soft – tissue compromise
that increases the risk of (or precludes) open intervention.
The author first explored this technique in cadavers and then
did a comprehensive literature review to see if anything similar
to BRAMIF had been developed previously. Two case reports and
one brief abstract from a poster presentation were found that
described the use of a balloon to aid in reduction of fractures
outside the spine. The first case was done in Japan using a
primitive urology balloon to aid in creating a void for
placement of fast-setting calcium phosphate in a comminuted
distal radius fracture with an arteriovenous fistula 42.
The authors of this case report referenced Jupiter et al.
28, who noted the benefits of calcium phosphate cement in
distal radius fractures, and were trying to find a way to insert
the calcium phosphate in an effective manner due to the
comminuted nature of their patient’s fracture with nearby
fistula. The second case report describes the percutaneous
insertion of a balloon for a nutcracker fracture in the cuboid
bone to aid in reduction. The authors noted good results using
an IBT for the cuboid reduction, but they used calcium sulfate
as a bone filler 43. The third
literature citation was an abstract for a poster presentation by
Reiley (co-founder of Kyphon, Inc.) 44
describing balloon-plasty of osteoporotic fractures of the
distal radius, distal femur, proximal tibia and calcaneus. This
brief abstract highlights several promising aspects of the use
of an IBT, including percutaneous reduction and cavity formation
with augmentation using minimal hardware. However, this
description of the percutaneous balloon-plasty technique makes
mention of only cancellous, osteoporotic fractures and no
guidance or specifics on technique were ever developed in the
extremity 44.
This paucity of literature has left a deficit of experience with
a potentially revolutionary technique for fracture treatment.
The authors believe that the principals of minimally invasive
or percutaneous surgery should be applied whenever possible in
order to minimize wound complications, and have found the BRAMIF
technique to be safe, reproducible, and valuable. Fractures in
areas such as the proximal tibia, distal tibia and calcaneous
are particularly prone to complications 5-10. The
BRAMIF technique described in this report provides an ideal
setting to support these periarticular, metaphyseal fractures
with a fast-setting calcium phosphate bone substitute. This
technique using an IBT, minimally invasive hardware, and calcium
phosphate bone filler is a logical evolution in extremity
fracture care.
The balloon acts in several important ways when used in this
manner. First, it acts as a reduction tool. This reduction
tool is superior to traditional methods of reduction in many
ways. Traditional bone tamps often require the surgeon to open
a split or window in the cortical bone. This can create more
damage and often limits the size of tamp that can be used to
lift up the compacted surfaces. The balloon, however, only
needs a small cannula (8 gauge, 4.0mm) to be inserted in the
cortical bone, and the defect or fracture can be easily reduced
as long as the balloon is inflated parallel to the joint
surface. This allows a much larger volume of bone to be lifted
up “en bloc” than the traditional bone tamp. Second, there is
no need to account for the often difficult angles needed to
insert a larger bone tamp, as even the largest IBT is inserted
through the same size cannula. Also, the surgeon can modify the
position of the balloon as needed for optimal fracture
reduction, even if the IBT is not perfectly parallel to the
joint line (see case example #1). Third, instead of just having
visual feedback (either fluoroscopy or straight visualization of
the joint line), there is an additional feedback of a pressure
gauge. We have found if the balloon is inserted into a
non-fractured area, then the balloon pressure increases very
rapidly and stays high (i.e. balloon is malpositioned and not
moving). In contrast, if the pressure continues to fall or
there is no increase in pressure then the void is too big or the
balloon is no longer contained within the bone (see “double
stacking” technique if void is too big). If the balloon is
placed correctly within a fractured area, the balloon pressure
increases but stays relatively low with an initial drop from
maximal pressure that levels out, allowing the surgeon to know
that the IBT is in the correct position. All these pressure
readings gives feedback that the surgeon cannot appreciate when
manually raising the bone with conventional bone tamps. With
enough experience, this feedback eliminates some of the need to
use fluoroscopy to monitor the fracture reduction. Finally,
just as the impaction of cancellous bone in the vertebral body
aids in keeping the PMMA inside the vertebral body 39,
45-47, the same is true at the articular surface. The
metaphyseal cancellous bone becomes compacted, which helps
contain the calcium phosphate within the bone without escaping
into the joint, as escape of cement from the vertebral body has
demonstrated detrimental effects in surrounding tissues 41,
48-49. However, just as in the kyphoplasty technique,
the void filler must still be watched with fluoroscopy closely
to ensure it does not leak into the joint line.
The IBT creates a well-defined bone void that facilitates the
delivery of fast-setting calcium phosphate cement for support of
the articular surface. We have found that temporary fixation
prior to balloon deflation is not usually necessary. Once the
balloon reduces the fragments, the fragments tend to stay
reduced, thereby allowing the surgeon to go straight to
permanent internal fixation after filling the void. In some
cases, hardware may be used to “frame” the fracture which acts
much like blocking screws that orthopaedic surgeons are used to
applying. The “frame” contains the balloon to the area where
the surgeon wants to create the bone void and achieve fracture
reduction. As an additional tip, even if doing a more
traditional open approach to any fracture reduction (e.g.
fragments are rotated or continued depression in spite of IBT
usage), the balloon can still be useful in impacting traditional
bone graft to achieve both support and a void to which other
bone fillers or additional graft can be applied.
The authors recommend using fast-setting calcium phosphate
cement as a bone void filler when using this technique.
Fast-setting calcium phosphate cements have several advantages
over other bone fillers. They are isothermic and set up quickly
in a wet environment 29, 50. The
calcium phosphate cement helps stabilize the fracture, providing
structurally competent augmentation with high compressive
strength that maintains its integrity while the cement is
resorbed, and is also osteoconductive so that it is replaced by
bone 27, 29-30, 36, 51. The advantages of calcium
phosphate cements have been shown clinically in multiple studies
31-34, 52. They decrease pain at the fracture site,
which may allow earlier mobilization 35, 53-57.
Three studies have demonstrated improved functional outcomes
with use of calcium phosphate cement 35, 57-59.
Several authors have demonstrated that calcium phosphate cements
are superior to traditional bone graft or no bone graft with
respect to preventing fracture subsidence 31, 35, 54-55,
57, 60. Also, by eliminating the need for
allograft there is no risk of potential shortage of cadaveric
bone material, patient objections, and allograft disease
transmission. With eliminating the need for autograft, there is
no donor site morbidity, which is often a problem 61-67.
There are other options
for bone fillers (excluding the prior mentioned allograft and
autograft), such as PMMA and calcium sulfates. PMMA is not
resorbable, and is highly exothermic as it sets 68-71,
which may be of concern in a subchondral location. One cannot
easily insert screws into PMMA 70-72. The
author has found the handling properties of calcium sulfate
products to be less desirable. Calcium sulfates are also a poor
choice because of their hydrophilic properties, lack of
structural support and quick resorption times 30, 73-74.
Due to their hydrophilic properties, a large amount of fluid may
accumulate in the area that often leads to wound healing and
infection problems 75-78. The authors believe that
the BRAMIF technique is not only applicable for osteoporotic
fractures, but may become a common treatment for acute fractures
of the distal radius, calcaneus, proximal and distal tibia.
There are additional properties of the IBT that makes it
beneficial to use in intra-articular extremity fractures with
the BRAMIF technique. The balloon, while performing the
reduction, generates a well-defined void to deliver and contain
the calcium phosphate cement. Also, the balloon indicates
exactly how much calcium phosphate will be needed by simply
reading the inflation gauge. The author developed this
technique not only from the concern of wound complications, but
also the struggles of getting calcium phosphate into the correct
position in the bone. Often, due to the high injection
pressures inside the bone, calcium phosphate would find cracks
and come out of the cortical shell. Worse yet, calcium
phosphate will also follow cracks through the articular
cartilage and diffuse into the joint. Instead, with use of the
IBT there is a void that readily receives the bone filler in the
exact position as the balloon was placed to give support to the
fracture at the articular surface, ideally where it is most
needed.
Economic costs are an important aspect of this technique as
well. Theoretically, the cost of treating one wound
complication is multiples more than the incremental expense of
the BRAMIF technique 1-2, 21, 79-81. This technique
also saves money by avoiding wastage of the typically expensive
bone void filler, since the volume of the void can be precisely
measured from the balloon(s). The incremental difference in
cost from using one volume of calcium phosphate cement to the
next larger volume (i.e. a 5cc to 10cc or a 10cc to a 15cc) is
typically more than the entire cost of an IBT system, and
surgeons without the balloon tend to err on the “safe” side, by
requesting the larger volume of filler. Overall, this novel
technique has a number of positive attributes that come at a
relatively small, or in some cases, negative economic cost.
This percutaneous technique allows reduction of fractures in a
manner that is minimally invasive while facilitating effective
application of ideal bone filler; perhaps more effectively than
is done with traditional open techniques in which there is a lot
of waste. These benefits may, in turn, provide a means to
perform reductions and internal fixations in patients that we
may have been rightfully hesitant to operate on in the past
(e.g. a diabetic, smoker with a joint-depression calcaneus
fracture, etc). This technique also allows placement of
fast-setting calcium phosphate cement in a reproducible manner,
directly beneath the impacted bone, with less likelihood of
intraarticular spillage or leaking out of the wound. Other
surgical approaches to these fractures require a delay while the
soft tissues settle down, often with temporary use of an
external fixator. Future research must be done to evaluate
whether the BRAMIF technique allows earlier definitive surgery
and leads to better outcomes, including earlier mobilization,
earlier discharge from the hospital, fewer surgeries and even
more overall cost savings. For now, these potential benefits
remain speculative.
Conclusion:
In conclusion, the authors report a novel technique, BRAMIF,
which utilizes an inflatable balloon bone tamp for reduction of
articular fractures, bone void creation, and insertion of
fast-setting calcium phosphate cement bone filler using a
minimally invasive approach. This technique may be readily
applied by orthopaedic surgeons to accomplish the list of goals
outlined herein. This includes a decrease in complications and
improving patient outcomes. We expect further refinements in
our technique as we and others gain experience with it. We
believe this already FDA-approved application of an inflatable
bone tamp can be learned and safely used by orthopaedic surgeons
who treat acute fractures of the distal radius, calcaneus, and
proximal and distal tibia. Hopefully, this report explains the
rationale and development of the BRAMIF technique and will lead
to further refinements in treatment of these difficult
fractures.
Reference :
-
The Burden of Musculoskeletal Diseases in the United States.
The Burden of Musculoskeletal Diseases in the United States.
2008; http://www.boneandjointburden.org. Accessed November 3,
2009.
-
The Burden of Musculoskeletal Diseases in the United States.
Musculoskeletal Injuries. The Burden of Musculoskeletal
Diseases in the United States 2008; http://www.boneandjointburden.org/pdfs/BMUS_chpt6_injuries.pdf.
Accessed November 3, 2009.
-
Garcia-Elias M, Folgar MA. The management of wrist injuries:
an international perspective. Injury. Nov
2006;37(11):1049-1056.
-
Dobriansky PJ, Suzman RM, Hodes RJ. Why Population Aging
Matters: A Global Perspective. 2007; http://www.nia.nih.gov/NR/rdonlyres/9E91407E-CFE8-4903-9875-D5AA75BD1D50/0/WPAM.pdf.
Accessed November 4, 2009.
-
Abidi NA, Dhawan S, Gruen GS, Vogt MT, Conti SF. Wound-healing
risk factors after open reduction and internal fixation of
calcaneal fractures. Foot Ankle Int. Dec
1998;19(12):856-861.
-
Bhattacharyya T, Crichlow R, Gobezie R, Kim E, Vrahas MS.
Complications associated with the posterolateral approach for
pilon fractures. J Orthop Trauma. Feb
2006;20(2):104-107.
-
Randle JA, Kreder HJ, Stephen D, Williams J, Jaglal S, Hu R.
Should calcaneal fractures be treated surgically? A
meta-analysis. Clin Orthop 2000;377:217-227.
-
Buckley R, Tough S, McCormack R, et al. Operative compared
with nonoperative treatment of displaced intra-articular
calcaneal fractures: a prospective, randomized, controlled
multicenter trial. J Bone Joint Surg Am. Oct
2002;84-A(10):1733-1744.
-
Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK.
Complications associated with internal fixation of high-energy
bicondylar tibial plateau fractures utilizing a two-incision
technique. J Orthop Trauma. Nov-Dec
2004;18(10):649-657.
-
Sirkin M, Sanders R, DiPasquale T, Herscovici D, Jr. A staged
protocol for soft tissue management in the treatment of
complex pilon fractures. J Orthop Trauma. Sep 2004;18(8
Suppl):S32-38.
-
Centers for Disease Control and Prevention. FastStats: Obesity
and Overweight. 2009; http://cdc.gov/nchs/fastats/overwt.htm.
Accessed November 3, 2009.
-
Centers for Disease Control and Prevention. Early Release of
Selected Estimates Based on Data From the January-March 2009
National Health Interview Survey. 2009; http://www.cdc.gov/nchs/nhis/released200909.htm.
Accessed November 2, 2009.
-
National Institute of Health. NIH Senior Health. 2009; http://nihseniorhealth.gov/diabetes/faq/faq3a.html.
Accessed November 2, 2009.
-
Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking
reduces incisional wound infection: a randomized controlled
trial. Ann Surg. Jul 2003;238(1):1-5.
-
Schmitz MA, Finnegan M, Natarajan R, Champine J. Effect of
smoking on tibial shaft fracture healing. Clin Orthop Relat
Res. Aug 1999(365):184-200.
-
Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk:
a meta-analysis. Osteoporos Int. Feb
2005;16(2):155-162.
-
Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its
complications and their relationship with risk of fractures in
type 1 and 2 diabetes. Calcif Tissue Int. Jan
2009;84(1):45-55.
-
Khazai NB, Beck GR, Jr., Umpierrez GE. Diabetes and fractures:
an overshadowed association. Curr Opin Endocrinol Diabetes
Obes. Dec 2009;16(6):435-445.
-
Chen H. "The relationship between obesity and injuries among
U.S. adults". Am J Health Promot. 2007;21(5):460-468.
-
Heron M, Hoyert D, Murphy S, Xu J, Kochanek K, Tejada-Wera B.
Deaths: Final Data for 2006. National Vital Statistics
Reports 2009; Volume 57, Number 14:http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf.
Accessed November 2, 2009.
-
Schneider EL, Guralnik JM. The aging of America. Impact on
health care costs. JAMA. May 2 1990;263(17):2335-2340.
-
Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and
safety of balloon kyphoplasty compared with non-surgical care
for vertebral compression fracture (FREE): a randomised
controlled trial. Lancet. Mar 21
2009;373(9668):1016-1024.
-
Garfin SR, Buckley RA, Ledlie J. Balloon kyphoplasty for
symptomatic vertebral body compression fractures results in
rapid, significant, and sustained improvements in back pain,
function, and quality of life for elderly patients. Spine (Phila
Pa 1976). Sep 1 2006;31(19):2213-2220.
-
Ledlie JT, Renfro MB. Kyphoplasty treatment of vertebral
fractures: 2-year outcomes show sustained benefits. Spine (Phila
Pa 1976). Jan 1 2006;31(1):57-64.
-
510(k). Summary of Safety and Effectiveness, Kyphon Inflatable
Bone Tamp. K981251. June 29 1998.
-
510(k). Summary of Safety and Effectiveness, KyphX Inflatable
Bone Tamp. K010246. January 25 2001.
-
Larsson S, Bauer TW. Use of injectable calcium phosphate
cement for fracture fixation: a review. Clinical
Orhtopaedics and Related Research. 2002;395:23-32.
-
Jupiter JB, Winters S, Sigman S, et al. Repair of five distal
radius fractures with an investigational cancellous bone
cement: a preliminary report. J Orthop Trauma. Feb-Mar
1997;11(2):110-116.
-
Frankenburg EP, Goldstein SA, Bauer TW, Harris SA, Poser RD.
Biomechanical and histological evaluation of a calcium
phosphate cement. J Bone Joint Surg Am. Aug
1998;80(8):1112-1124.
-
Hak DJ. The use of osteoconductive bone graft substitutes in
orthopaedic trauma. J Am Acad Orthop Surg.
2007;15(9):525-536.
-
Keating JF, Hajducka CL, Harper J. Minimal internal fixation
and calcium-phosphate cement in the treatment of fractures of
the tibial plateau. A pilot study. J Bone Joint Surg Br.
Jan 2003;85(1):68-73.
-
Thordarson DB, Hedman TP, Yetkinler DN, Eskander E, Lawrence
TN, Poser RD. Superior compressive strength of a calcaneal
fracture construct augmented with remodelable cancellous bone
cement. J Bone Joint Surg Am. Feb 1999;81(2):239-246.
-
Wee AT, Wong YS. Percutaneous reduction and injection of
Norian bone cement for the treatment of displaced intra-articular
calcaneal fractures. Foot Ankle Spec. Apr
2009;2(2):98-106.
-
Simpson D, Keating JF. Outcome of tibial plateau fractures
managed with calcium phosphate cement. Injury. Sep
2004;35(9):913-918.
-
Bajammal SS, Zlowodzki M, Lelwica A, et al. The use of calcium
phosphate bone cement in fracture treatment. A meta-analysis
of randomized trials. J Bone Joint Surg Am. Jun
2008;90(6):1186-1196.
-
Yetkinler DN, Ladd AL, Poser RD, Constantz BR, Carter D.
Biomechanical evaluation of fixation of intra-articular
fractures of the distal part of the radius in cadavera:
Kirschner wires compared with calcium-phosphate bone cement.
J Bone Joint Surg Am. Mar 1999;81(3):391-399.
-
Stryker. R & D Test Report TR-1808. http://www.stryker.com/en-us/products/Orthopaedics/BoneSubstitute/Hydroset/index.htm.
Accessed November 3, 2009.
-
Larsson S. Injectable phosphate cements - A review. Uppsala,
Sweden2006:1-12.
-
Garfin SR, Yuan HA, Reiley MA. New technologies in spine:
kyphoplasty and vertebroplasty for the treatment of painful
osteoporotic compression fractures. Spine (Phila Pa 1976).
Jul 15 2001;26(14):1511-1515.
-
Yeom JS, Kim WJ, Choy WS, Lee CK, Chang BS, Kang JW. Leakage
of cement in percutaneous transpedicular vertebroplasty for
painful osteoporotic compression fractures. J Bone Joint
Surg Br. Jan 2003;85(1):83-89.
-
Lin EP, Ekholm S, Hiwatashi A, Westesson PL. Vertebroplasty:
cement leakage into the disc increases the risk of new
fracture of adjacent vertebral body. AJNR Am J Neuroradiol.
Feb 2004;25(2):175-180.
-
Ishiguro S, Oota Y, Sudo A, Uchida A. Calcium phosphate
cement-assisted balloon osteoplasty for a Colles' fracture on
arteriovenous fistula forearm of a maintenance hemodialysis
patient. The Journal of Hand Surgery.
2007;32(A):821-826.
-
Heim KA, Sullivan C, Parekh SG. Cuboid reduction and fixation
using a kyphoplasty balloon: a case report. Foot Ankle Int.
2008;29(11):1154-1157.
-
Reiley MA. Percutaneous balloon-plasty technique and results
for tibial plateau, distal radius, femoral condylar, and
calcaneal fractures. J Orthop Trauma. 2003;17(2):161.
-
Gaitanis IN, Hadjipavlou AG, Katonis PG, Tzermiadianos MN,
Pasku DS, Patwardhan AG. Balloon kyphoplasty for the treatment
of pathological vertebral compressive fractures. Eur Spine
J. Apr 2005;14(3):250-260.
-
Dudeney S, Lieberman IH, Reinhardt MK, Hussein M. Kyphoplasty
in the treatment of osteolytic vertebral compression fractures
as a result of multiple myeloma. J Clin Oncol. May 1
2002;20(9):2382-2387.
-
Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome
and efficacy of "kyphoplasty" in the treatment of painful
osteoporotic vertebral compression fractures. Spine (Phila
Pa 1976). Jul 15 2001;26(14):1631-1638.
-
Heo DH, Cho SM, Cho YJ, Cho JH, Sheen SH. Heterotopic
ossifications after vertebroplasty using calcium phosphate in
osteoporotic vertebral compression fractures Report of 2
cases. Surg Neurol. Oct 12 2009.
-
DalCanto RA, Reinhardt MK, Lieberman IH. Double
cement-application cavity containment kyphoplasty: technique
description and efficacy. Am J Orthop. Jul
2009;38(7):E110-114.
-
Report ST. Stryker Test Report #1808. http://www.stryker.com/en-us/products/Orthopaedics/BoneSubstitutes/Hydroset/index.htm.
-
Stevenson S. Biology of bone grafts. Orthop Clin North Am.
Oct 1999;30(4):543-552.
-
Johal HS, Buckley RE, Le IL, Leighton RK. A prospective
randomized controlled trial of a bioresorbable calcium
phosphate paste (alpha-BSM) in treatment of displaced intra-articular
calcaneal fractures. J Trauma. Oct 2009;67(4):875-882.
-
Higgins TF, Dodds SD, Wolfe SW. A biomechanical analysis of
fixation of intra-articular distal radial fractures with
calcium-phosphate bone cement. J Bone Joint Surg Am.
Sep 2002;84-A(9):1579-1586.
-
Welch RD, Zhang H, Bronson DG. Experimental tibial plateau
fractures augmented with calcium phosphate cement or
autologous bone graft. J Bone Joint Surg Am. Feb
2003;85-A(2):222-231.
-
Sanchez-Sotelo J, Munuera L, Madero R. Treatment of fractures
of the distal radius with a remodellable bone cement: a
prospective, randomised study using Norian SRS. J Bone
Joint Surg Br. Aug 2000;82(6):856-863.
-
Dickson KF, Friedman J, Buchholz JG, Flandry FD. The use of
BoneSource hydroxyapatite cement for traumatic metaphyseal
bone void filling. J Trauma. Dec 2002;53(6):1103-1108.
-
Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement
compared with conventional fixation in distal radial
fractures. A randomized study. J Bone Joint Surg Am.
Nov 2003;85-A(11):2127-2137.
-
Zimmermann R, Gabl M, Lutz M, Angermann P, Gschwentner M,
Pechlaner S. Injectable calcium phosphate bone cement Norian
SRS for the treatment of intra-articular compression fractures
of the distal radius in osteoporotic women. Arch Orthop
Trauma Surg. Feb 2003;123(1):22-27.
-
Mattsson P, Alberts A, Dahlberg G, Sohlman M, Hyldahl HC,
Larsson S. Resorbable cement for the augmentation of
internally-fixed unstable trochanteric fractures. A
prospective, randomised multicentre study. J Bone Joint
Surg Br. Sep 2005;87(9):1203-1209.
-
Trenholm A, Landry S, McLaughlin K, et al. Comparative
fixation of tibial plateau fractures using alpha-BSM, a
calcium phosphate cement, versus cancellous bone graft. J
Orthop Trauma. Nov-Dec 2005;19(10):698-702.
-
Silber JS, Anderson DG, Daffner SD, et al. Donor site
morbidity after anterior iliac crest bone harvest for
single-level anterior cervical discectomy and fusion.
Spine. Jan 15 2003;28(2):134-139.
-
Auleda J, Bianchi A, Tibau R, Rodriguez-Cano O. Hernia through
iliac crest defects. A report of four cases. Int Orthop.
1995;19(6):367-369.
-
Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous
iliac crest bone graft. Complications and functional
assessment. Clin Orthop Relat Res. Jun 1997(339):76-81.
-
Kurz LT, Garfin SR, Booth RE, Jr. Harvesting autogenous iliac
bone grafts. A review of complications and techniques.
Spine (Phila Pa 1976). Dec 1989;14(12):1324-1331.
-
Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA.
Complications of iliac crest bone graft harvesting. Clin
Orthop Relat Res. Aug 1996(329):300-309.
-
Summers BN, Eisenstein SM. Donor site pain from the ilium. A
complication of lumbar spine fusion. J Bone Joint Surg Br.
Aug 1989;71(4):677-680.
-
Younger EM, Chapman MW. Morbidity at bone graft donor sites.
J Orthop Trauma. 1989;3(3):192-195.
-
Santin M, Motta A, Borzachiello A, Nicolais L, Ambrosio L.
Effect of PMMA cement radical polymerisation on the
inflammatory response. J Mater Sci Mater Med. Nov
2004;15(11):1175-1180.
-
Radev BR, Kase JA, Askew MJ, Weiner SD. Potential for thermal
damage to articular cartilage by PMMA reconstruction of a bone
cavity following tumor excision: A finite element study. J
Biomech. May 29 2009;42(8):1120-1126.
-
Orr JF, Dunne NJ, Quinn JC. Shrinkage stresses in bone cement.
Biomaterials. Aug 2003;24(17):2933-2940.
-
Chu KT, Oshida Y, Hancock EB, Kowolik MJ, Barco T, Zunt SL.
Hydroxyapatite/PMMA composites as bone cements. Biomed
Mater Eng. 2004;14(1):87-105.
-
Hingston JA, Dunne NJ, Looney L, McGuinness GB. Effect of
curing characteristics on residual stress generation in
polymethyl methacrylate bone cements. Proc Inst Mech Eng H.
Aug 2008;222(6):933-945.
-
Thomas MV, Puleo DA. Calcium sulfate: Properties and clinical
applications. J Biomed Mater Res B Appl Biomater. Feb
2009;88(2):597-610.
-
Hing KA, Wilson LF, Buckland T. Comparative performance of
three ceramic bone graft substitutes. Spine J. Jul-Aug
2007;7(4):475-490.
-
Catalano PJ, Insley G, Hess B. An in-vivo comparative analysis
of the intra-operative properties of injectable calcium
phosphate/calcium sulfate based bone cements. Key
Engineering Materials. 2007;330-332:799-802.
-
Robinson D, Alk D, Sandbank J, Farber R, Halperin N.
Inflammatory reactions associated with a calcium sulfate bone
substitute. Ann Transplant. 1999;4(3-4):91-97.
-
Lee GH, Khoury JG, Bell JE, Buckwalter JA. Adverse reactions
to OsteoSet bone graft substitute, the incidence in a
consecutive series. Iowa Orthop J. 2002;22:35-38.
-
Lewis KN, Thomas MV, Puleo DA. Mechanical and degradation
behavior of polymer-calcium sulfate composites. J Mater Sci
Mater Med. Jun 2006;17(6):531-537.
-
de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn
BB. Surgical site infection: incidence and impact on hospital
utilization and treatment costs. Am J Infect Control.
Jun 2009;37(5):387-397.
-
Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton
DJ. The impact of surgical-site infections following
orthopedic surgery at a community hospital and a university
hospital: adverse quality of life, excess length of stay, and
extra cost. Infect Control Hosp Epidemiol. Apr
2002;23(4):183-189.
-
Borgstrom F, Zethraeus N, Johnell O, et al. Costs and quality
of life associated with osteoporosis-related fractures in
Sweden. Osteoporos Int. 2006;17(5):637-650.
|












