|
Abstract:
Objective
Ovariectomy(OVX) can cause bone loss in rats, but little is
known about the relationship of osteoporosis(OP) and
osteoarthritis(OA) .This study investigated how OA affects the
development of OP in OVX rats. Merterials and Methods
Fifty 3-month-old female Sprague-Dawley rats were divided
randomly into five equal groups. The baseline control group (BL
group) was euthanized at the beginning of the experiment. A
bilateral ovariectomy was performed in 10 rats (OVX group) and
another group of 10 rats was subjected to a sham surgery (Sham
group). Osteoarthritis was induced by transection of the
anterior cruciate ligament of the right knee in 10 rats (OA
group). Bilateral ovariectomy and transection of the anterior
cruciate ligament of the right knee was preformed in the last
group (OVX+OA group). Bone mineral density (BMD) measurement and
bone histomorphometric analysis were applied to the right femora
in all rats evaluated 6 months after surgery. Before death, they
were all double-labeled with subcutaneous injections of
tetracycline and calcein. Results The bone mass of the
OVX group is significantly lower than that of the Sham group (P
< 0.05). The bone mass of the OVX+OA group is significantly
higher than that of the OVX group. The bone mass of the OVX+OA
group is significantly lower than that of the OA group. There is
no significant difference in bone mass between the OA and Sham
groups. Conclusion In conclusion, OA retards the
development of OP in the distal femur of OVX rats.
J.Orthopaedics 2010;7(1)e3
Keywords:
Osteoporosis; Osteoarthritis; Bone histomorphometry; Ovariectomy
Introduction:
Primary
osteoporosis (OP) often occurs in the elderly, especially
postmenopausal women. Many risk factors are associated with
primary OP, such as smoking, hormone condition, menstrual
history, and genetic risk factors. The reduction in estrogen
production is thought to be responsible for bone loss in
postmenopausal OP patients 1. Primary osteoarthritis
(OA) is a prevalent chronic degenerative joint disease and it
most often affects the joints of limbs such as the knee and hip.
Some studies have observed that primary OP and primary OA in
peripheral joints do not often occur in the same patient, and an
inverse relationship was indicated between these two common
diseases 2, 3. However, previous studies of the
relationship between OP and OA did not exclude the influences of
these risk and genetic factors such that these confounding
factors possibly influenced the assessment. Therefore, until
now, it remains debatable whether OA retards the development of
OP4, 5. The use of animal models is a powerful tool
for understanding the similarities and differences between them
and the human case and for providing preclinical references for
the diagnosis and treatment of diseases. Moreover, they can
reduce the influences of certain confounding factors that exist
in humans but not in experimental animals6,7.To our
knowledge, few animal models have been used for the study of the
relationship between OP and OA. Therefore, the purpose of this
study was to examine the effects of OA and estrogen deficiency
on the distal femoral in OVX rats, widely used as models to
simulate the estrogenic condition of postmenopausal women, and
to investigate the relationship between the two common diseases
in animal model.
Materials
and Methods:
Experimental Design
Fifty
3-month-old female Sprague-Dawley rats (Peking University Animal
Center), weighing approximately 210±32 g at the beginning of the
experiment were randomized into five groups of 10 animals each.
The rats were housed in individual cages and were given food and
water ad libitum during the experimental period. The rats in the
baseline group (BL group) were killed at the beginning of the
study. Under 1% pentobarbital sodium 40 mg/kg anesthesia,
bilateral ovariectomy was performed on 10 rats through a dorsal
approach under sterile conditions (OVX group). Another 10 rats
were subjected to sham surgery in which the ovaries were exposed
but not removed (Sham group) 8,9. Osteoarthritis was
induced by transection of the anterior cruciate ligament of the
right knee in 10 rats (OA group). Bilateral ovariectomy and
transection of the anterior cruciate ligament of the right knee
in the fifth group(OVX+OA group) 10. All the rats
were sacrificed 6 months after the operation (9 months old). All
the animals received fluorochrome double-labeling with
subcutaneous injections of 30 mg/kg of tetracycline (Shanghai
Xinya Pharmaceutical Factory) and 6 mg/kg of calcein (Sigma
Chemical Co. St. Louis, MO) on the tenth day and the third day,
respectively, before euthanasia. When soft tissues were
carefully removed, the right femur was sawed into 4 equal parts
with a low-speed metallurgical saw (Buehler LTD.USA) to take
into account of possible differences in the local proportion of
trabecular and cortical bone, distal femur, proximal femur,
midshaft femur (The two equal regions of diaphysis were
considered). The distal parts that we needed were performed BMD
measurement with dual–energy X-ray absorptiometry (Norland
XR-36, USA) adapted to measuring small objects. Reproducibility,
as a coefficient of variation (CV) from five measurements of the
same femora after respositioning, was 0.52%. The stability of
the instrument was controlled by scanning a phantom two times a
week11.
Specimen Preparation and Staining for histomorphometric
measurements
The
distal parts were trimmed at frontal view to expose the marrow
cavity for better fixation and used for the histomorphometric
measurement. The distal part of the right femora were fixed with
70% ethanol and then dehydrated in ascending grades of ethanol,
defatted in an acetone ethanol mixture (1:1), and embedded in
methyl methacrylate without decalcification. The frontal
sections were cut at 4 and 8 μm thickness with a microtome (Leica
RM 2155, Germany). The 4 μm sections were stained with Goldner’s
Trichrome for bone static and cell parameter measurements, and
the unstained 8 μm sections were used for dynamic
histomorphometric analysis of fluorochrome labeling.
Histomorphometric Analysis
Histomorphometric measurements were performed by an independent
individual who was unaware of the experimental protocol, using a
digitizing image analysis system (DIAS; KSS Image, Magna, UT,
USA) which consists of a light and an epifluorescent microscope
coupled to a computer with a morphometry program“stereology”.The
measurement site on the bone section was between 1 and 4 mm
distal to the growth plate–epiphyseal line and bilaterally
between the endocortical envelope. The histomorphometric
terminology for cancellous bone measurements was employed
according to the methods described by Cui liao et al. [12]
Statistical Analysis
Data
were analyzed using the SAS system (Ver. 6.12; SAS Institute,
Cary, NC). All descriptive data were expressed as mean ± SD.
Student’s t-test and Wilcoxon test for independent nonparametric
samples were applied to compare significant differences between
the two groups. A two-sided probability value of P < 0.05
was considered statistically significant. The values of the BL
group were provided as a reference for comparison but were not
included in the histomorphometric analysis.
Results :
Effects of OVX, OA, OVX+OA on distal femoral BMD ( Table 1)
Table
1. BMD values among the five groups (g/cm2)
|
Groups |
Distal femoral |
|
BL |
0.0856±0.0214 |
|
Sham |
0.1335±0.0098 |
|
OVX |
0.1087±0.0076a |
|
OA |
0.1303±0.0072 b |
|
OVX+OA |
0.1184±0.0066,a,b,c |
Data
are the mean ± SD of 10 values per group
a p<0.05,significantly different from the
time-corresponding Sham group.
b p<0.05,significantly different from the
time-corresponding OVX group.
c p<0.05,significantly different from the
time-corresponding OA group.
The BMD
of the distal femoral in the OVX group increased in comparison
with the BL group but significantly decreased compared with the
Sham group. There were no differences in BMD between the OA and
Sham groups. The BMD of the OVX+OA group were significantly
higher than those of the OVX group. The BMD of the OVX+OA group
were significantly lower than those of the OA group.
Effects of OVX, OA, OVX+OA on distal femoral metaphyseal
histomorphometry ( Table 2&3)
Table
2. Static histomorphometric indices of cancellous bone in the
distal femoral in the four groups
|
Parameters BV/TV( %) Tb.N (mm-1)
Tb.Th (μm) Tb.Sp (μm) Oc. No/BV (mm-2)
Oc. Pm/BS (%) |
|
Sham
15.86±3.76 2.14±0.63 74.79±3.84
419.77±108.75 6.33±1.00
0.33 ± 0.02
OVX
3.32±1.03a 0.46±0.11a
71.60±12.31 2208.80±530.46a
13.72±4.36a 0.68 ± 0.09 a
OA
14.36±2.09 b 1.81±0.23 b 79.33±8.74
479.37±66.83 b 6.24±1.32
b 0.31 ± 0.04 b
 OVX+OA
6.76±0.68a,b,c 0.84±0.06a,b,c
80.75±8.74 1116.46±78.12a,b,c 9.71±1.69a,b,c
0.47 ± 0.06 a,b,c OVX+OA
6.76±0.68a,b,c 0.84±0.06a,b,c
80.75±8.74 1116.46±78.12a,b,c 9.71±1.69a,b,c
0.47 ± 0.06 a,b,c
|
Data
are the mean ± SD of 10 values per group
a p<0.05,significantly different from the
time-corresponding Sham group.
b p<0.05,significantly different from the
time-corresponding OVX group.
c p<0.05,significantly different from the
time-corresponding OA group.
Table
3. Dynamic histomorphometric indices of cancellous bone in the
distal femoral in the four groups
|
Parameters sL. Pm (mm) dL.Pm (mm) MS/BS (%) MAR (μm
/day) BFR/BS (μm /day*100) BFR/BV |
|
Sham 2.21 ± 0.4 0.92 ± 0.09 5.42 ± 1.07
1.15±0.18 8.47±2.36
69.23±19.99
OVX 1.41 ± 0.19 0.54 ± 0.18a
8.76 ± 2.83a
1.48±0.16a
19.69±6.53a 170.56±60.93a
OA 2.18 ± 0.5 0.97 ± 0.12 b
5.68 ± 1.03 b
1.05±0.14
b 7.28±0.88 b 56.19±6.89 b
OVX+OA 1.39 ± 0.15 0.75 ± 0.14a,b,c 7.49 ±
2.42a,c 1.32±0.10b,c 12.41±1.05a,b,c
94.06±8.59a,b,c |
Data
are the mean ± SD of 10 values per group
a p<0.05,significantly different from the
time-corresponding Sham group.
b p<0.05,significantly different from the
time-corresponding OVX group.
c p<0.05,significantly different from the
time-corresponding OA group.
Compared with the Sham group, some static histomorphometric
indices of cancellous bone in the OVX group were significantly
lower (trabecular bone volume and trabecular number), whereas
indices of trabecular separation, osteoclasts number–bone volume
referent, and percent osteoclast surface were significantly
higher. Similarly, while some dynamic indices were significantly
higher than in the Sham group (indices of mineralizing
surface–bone surface referent and bone formation rate), the
double-labeled perimeter was significantly lower. There were no
significant differences in all the histomorphometric indices
between the OA and Sham groups. The Tb.Sp, Oc.No/BV, Oc.Pm/BS,
MAR, BFR/BS, BFR/BV of the OVX+OA group were significantly lower
than those of the OVX group, but the BV/TV, Tb.N, dL.Pm were
significantly higher. The Tb.Sp, Oc.No/BV, Oc.Pm/BS, MAR, BFR/BS,
BFR/BV of the OVX+OA group were significantly higher than those
of the OA group, but the BV/TV, Tb.N, dL.Pm were significantly
lower.
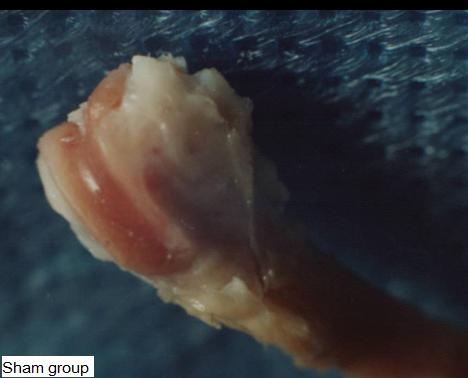
Figure 1: The surface of the distal femoral in the Sham
group (9 months old) was very smooth, had the gloss, no
articular cartilage destruction and no osteophyte formation on
the edge of the femoral condyle.
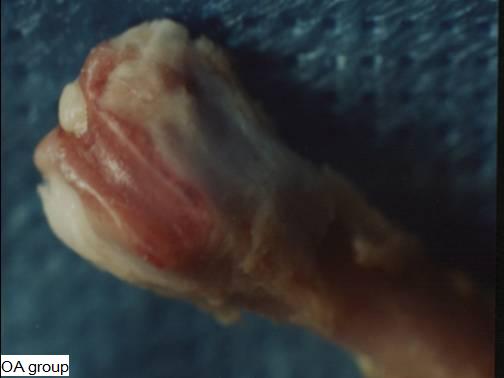
Figure 2: The surface of the distal femoral in the OA group
(9 months old) was much coarser than that in the Sham group.
There were articular cartilage destruction and osteophytes
formation on the edge of the femoral condyle. The degeneration
of the knee indicated formation of the OA.
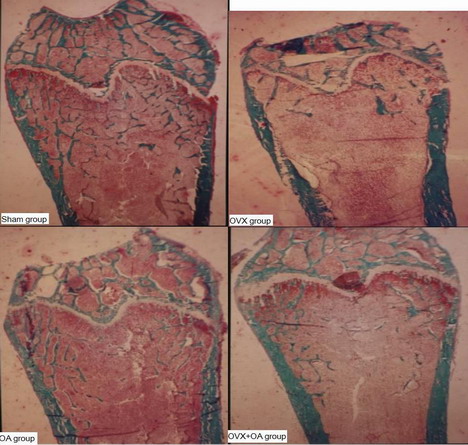
Figure 3: These pictures showed the structures of metaphyses,
trabecular bone and epiphyseal line et.al. on distal femoral in
Sham group, OVX group, OA group and OVX+OA group rats. The white
line on the upper part of the picture was epiphyseal line, the
green funicular structures below that were trabecular bone. The
measurement site on the bone section was between 1 and 4 mm
distal to the growth plate–epiphyseal line and bilaterally
between the endocortical envelope. The pictures showed that the
trabecular number of the OVX group was significantly lower than
that of the Sham group, the trabecular number of the OVX+OA
group was significantly higher than that of the OVX group, the
trabecular number of the OVX+OA group was significantly lower
than that of the OA group (Masson-Goldner Trichrome staining 4
μm sections without decalcification, original magnification
10×).
Discussion :
In our
data the rats had remarkable bone loss and high bone turnover 6
months after the ovariectomy. The OVX-induced bone loss was due
to an increase in bone resorption caused by estrogen deficiency
accompanied by an increase in bone formation that was
insufficient to compensate for the increase in resorption
thereby leading to bone loss. Our results demonstrated that we
duplicated a postmenopausal OP animal model 13.
Joint
instability was a well known cause of secondary OA of the human
knee. To study the pathogenesis of instability induced OA,
animal models were frequently used. There was a consistent
relationship between the development of OA and knee instability
as a result of transection of the anterior cruciate ligament in
knees of animal models such as the rat[10]. In this
study, the surfaces of the distal femoral in OA group were much
coarser than that in the non-OA group. The degeneration of the
knee indicated formation of the OA.
In
clinical application, there were three different viewpoints on
the relationship between OP and osteoarthritis OA14,15.
The first was that OA retards the development of OP. The second
was OA accelerates the development of OP. The third was these
two diseases were not correlative in pathology process.
This
study demonstrated that: 1)The bone mass of the OVX+OA group is
significantly higher than that of the OVX group. There was a
decrease in bone formation related parameters such as MAR. The
bone resorption parameters as Oc.No/BV, Oc.Pm/BS and bone
turnover (BFR/BV) were also decreased. The OVX+OA group rats
could prevent the OVX-induced cancellous bone loss by a decrease
in bone turnover. Our findings suggested that OVX+OA group rats
created a positive bone balance compare with the OVX group rats.
2) The bone mass of the OVX+OA group was significantly lower
than that of the OA group. There was an increase in both the
bone resorption related parameter such as Oc.No/BV, Oc.Pm/BS and
bone formation related parameter as MAR. Due to the bone
turnover parameter (BFR/BV) in the OVX+OA group was
significantly higher than that of the OA group, the cancellous
bone mass were lossed.3)There was no significant differences in
bone mass between the OA and Sham groups. That was because the
bone formation and resorption parameters in the OA and Sham
groups have no difference.
In
other clinical research, Verstraeten et al.4 found
that the osteoarthrotic patients had fewer forearm and other
fractures compared with the OP patients, while the OP patients
had a significantly lower degree of osteoarthrosis in the hand,
hip joints, and spine compared with the osteoarthrotic patients.
Therefore, we hypothesize that primary OA might have a
protective effect on the progression of OP with its related
factors, such as high levels of insulin like growth factor,
being overweight, decreased bone turnover, and genetics.
In
summary, OA retards the development of OP on the distal part of
the femur in OVX rats.
Conclusion:
Reference :
-
Stevenson JC, Lees B, Devenport M, Cust MP, Ganger
KF Determinants of bone density in normal women: risk factors
for future osteoporosis? Br Med J 1989; 298:924-928
-
Weintroub S, Papo J, Ashkenazi M, Tardiman R, Weissman SL,
Salama R Osteoarthritis of hip and fractures of the proximal
end of the femur.
Acta Orthop Scand
1982;
53:261-264
-
Marcelli C, Favier F, Kotzki PO, Ferrazzi V, Picot MC,
Simon L The relationship between osteoarthritis of the hands,
bone mineral density, and osteoporotic fractures in elderly
women. Osteoporosis International 1995; 5:382-388
-
Verstraeten A, Van Ermen H, Haghebaert G, Nijs J, Geusens P,
Dequeker J Osteoarthrosis retards the development of
osteoporosis: observation of the coexistence of Osteoarthrosis
and osteoporosis. Clinical Orthopaedics and Related Research
1991; 264:169-177
-
Miyakoshi N, Itoi E, Murai H, Wakabayashi I, Ito H, Minato T
Inverse relation between osteoporosis and spondylosis in
postmenopausal women as evaluated by bone mineral density and
semiquantitative scoring of spinal degeneration. Spine 2003;
28:492-495
-
Zhang L, Endo N, Yamamoto N Effects of single and concurrent
intermittent administration of human PTH(1-34) and incadronate
on cancellous and cortical bone of femoral neck in
ovariectomized rat. Tohoku J.Exp.Med 2003; 186:131-141
-
Li QN, Liang NC, Huang LF Skeletal effects of constant and
terminated use of sodium risedronate in ovariectomized rats.
Acta Pharmacologica Sinica 1998; 19:160-163
-
Zhang L, Takahashi HE, Inoue J Effects of intermittent
administration of low dose human PTH(1-34) on cancellous and
cortical bone of lumbar vertebral bodies in adult beagles.
Bone 1997; 21:501-506
-
Li QN, Jee WSS, Ma YF Risedronate pretreatment does not
hamper the anabolic effects of prostaglandin E2 in OVX rats.
Bone 1995; 17:261-266.
-
Williams JM, Felton DL, Peterson RG Effects of surgically
induced instability on rat knee articular cartilage. J Anat
1982; 134:103-109.
-
Wang T, Zhang L, Huang C, Cheng AG, Dang GT
Relationship between osteopenia and lumbar intervertebral disc
degeneration in ovariectomized rats. Calcified Tissue
International
2004;
75:205-213.
-
Cui L, Wu T, Liu XQ, Li QN, Lin LS Preventive effects of
ginsenosides on osteopenia of rats induced by ovariectomy.
Acta Pharmacol Sin 2001; 22:428-434.
-
Kalu DN The ovariectomized rat model of postmenopausal bone
loss. Bone and Mineral 1991; 15:175-192
-
Ding RK, Sun CJ, Wang WC Relationships of the osteoarthritis
of the knee joint with the osteoporosis. Hunan Medical Journal
1997; 14:323-324
-
Dequeker J The relationships between osteoporosis and
osteoarthritis. Clin Rheum Dis 1985; 11:271-274
|





