|
Abstract:
The use of biomaterials is continuously increasing in daily
orthopaedic practice. New materials are introduced in order to
combine as many as possible favourable properties in one and
only material. Porous tantalum has unique characteristics. Its
elasticity is close to subchondral bone, while at the same time
it is highly adherent. It has a great porosity of up to 80% of
its total volume which promotes osteointegration. It can be
constructed in a variety of sizes and shapes that allows its
application in many areas of orthopaedic surgery.
J.Orthopaedics 2009;6(4)e3
Keywords:
porous tantalum;
interface;
acetabular implant
Introduction:
During the
last 25 years various biomaterials and porous surfaces have been
used in the area of reconstructive orthopaedic surgery, in order
to achieve osteo-integration
of the implants. Clinical and histological data from removed
parts of arthroplasties advocate that porous surfaces reinforce
the implant’s stability via biological integration procedures.
However, the most common used materials, titanium and
cobalt-chromium alloy, can’t respond to the demanding mechanical
status of orthopaedic implants because of their mechanical
features. This is the reason why they are used only as modes of
superficial coating, while the implant’s supporting structure is
made from another compact metal. Also, the rate of porous
texture of these coatings is about 30%-50% of their total volume
and this restricts their ability of bony penetration. The latter
is proportional to the amount of pores, expressed as percentage
of the total volume of the material1-3. In the area
of structural grafts at the same time, a large number of
synthetic biomaterials have been introduced and significant
improvements in use of bone allografts have been made. The
purpose of all these is the restoration of bone deficit areas
with simultaneous bone penetration and integration. The
biomaterial’s ability to succeed this, depends mainly on the
porous texture, the mechanical resistance, both short and long
term, the elasticity (which ideally should approach the
elasticity of cancellous bone), the resistance to absorption,
the immune inactivity and the ease of manufacturing and use. It
is obvious that the combination of a high ratio of porous
texture, with the appropriate mechanical properties for use in
orthopaedic surgery in one and only biomaterial is particularly
desirable.
Properties of pure tantalum
Pure tantalum (ASTM F-560) is a highly biocompatible material
which does not provoke cellular reactions as other materials
like nickel, cobalt and chromium4,5 do. It is a hard
metal which resists in oxidation, corrosion and concomitant ion
production6. Tantalum is being used for over 50 years
as an implant in humans, in a variety of applications:
electrodes for pacemakers7, plates for cranioplasty8
, clips for ligation9 , femoral stems10,
wires, nets or plates in nerve surgery11 and as a
medium of marking in radiological studies for implant migration12.
The property of tantalum to adhere to bone, possibly via a not
well elucidated chemical connection procedure, is known for many
years, mainly through the application of tantalum in the area of
dental implantation13. Historically, only the pure
titanium and tantalum have this property that also newer
biomaterials (CaHAP, crystalloids, hyaloids) have. According to
Kokubo et al14, after the exposition of tantalum in
body moist, a superficial layer of sodium tantalum is created,
ions of Na+ are released from this and connections of
tantalum with hydroxyl roots are made (Ta-OH). The latter are
connected with free Ca ions (Ca++) and thus amorphous
calcium tantalum is created (Calcium Tantalate). The combination
of calcium tantalum with phosphorus ions leads to amorphous
phosphoric calcium, which is converted to hydroxyapatite. In
this way, a solid connection between bone and metal is achieved.
Recently, Findlay and Whelldon15,16 tested the
ability of polished tantalum to support the growth and function
of normal human osteoblastic cells, in comparison with other
substrates and specifically titanium and chromium-cobalt (that
have proved in clinical practice their ability for bony
integration). They studied the number and morphology of adherent
osteoblasts with electronic microscopy and their rate of
proliferation and biological activity, expressed via mRNA
concentrations. They concluded that pure tantalum is at least
the same effective with the above mentioned biomaterials as a
substrate for adhesion, growth, differentiation and function of
human osteoblasts.
Porous tantalum preparation and
properties
Porous tantalum is a new biomaterial, which was constructed a
decade ago17,18. Preparation of porous tantalum
starts with polyurethane’s foam pyrolysis (thermal conversion).
This foam turns to a skeleton of low density hyaloid carbon,
with a characteristic repeated duodenofundament structure that
leads to pores connected with smaller holes (Fig 1). By the
method of chemical vapor deposition-infiltration (CVD/CVI),
metal tantalum deposition is taking place on carbon skeleton and
a porous metal construction is formed. Due to crystallographic
processes and the tantalum’s orientation during the deposition,
this procedure leads to the creation of a surface with special
texture, similar to the cancellus bone. The mean pore’s diameter
is 547+/- 52μm.
Pores are connected with smaller holes. The pore’s
two-dimensional size is 430 +/- 270
μm.
The porosity is about 75%-80% of the total material’s volume.
Pore’s size is ideal for vascular tissue ingrowth. The large
amount of empty space in this material allows deep and extensive
tissue penetration (Fig 2) and this results to stronger adhesion
and biological fixation19. This geometry also allows
the penetration of non-biological materials, as polyethylene,
that can be press - molded onto shell leading to a strong
monoblock construction. Moreover, this allows, potentially, the
deposition of osteoinductive and osteoconductive factors20
which promote the material’s bone integration. The thin coating
layer from tantalum at the initial scaffold, with thickness 10μm-100μm,
can give high mechanical properties, because this deposition has
100% density, the granule’s metal size is less than 1-5μm
and other’s material mixing is less than 0,05%. The typical mean
thickness of tantalum coating is 50
μm.
This microarchitecture possibly participates in the overall
osteogenic response, as has been proven in the past from studies
in cell cultures with materials21 of similar
microarchitecture.
Porous tantalum has also favorable elasticity and friction ratio21.
Biomaterial’s elasticity is 3GPa, very close to subchondral
bone, and this permits the normal load transferring from implant
to the surrounding cancellous bone. This is an important factor
for implant’s long-lasting survival. Tantalum’s resistance at
compression is 50-80 Mpa, almost like cancellus bone, and the
resistance at rotational deformity is 40-60Mpa. The resistance
threshold in traction is 18-20Mpa. As a result, porous tantalum
has a high degree of plasticity in compression without failure,
and can afford the usual loads of total hip arthroplasty or
while supporting the femoral head as a rod in cases of femoral
avascular necrosis. The same happens in the area of bone grafts,
where resistance at compression-traction-bending appears in ex
vivo studies to be larger than cancellous bone’s and others
metal scaffold’s resistance. This supports the hypothesis of a
more stable mechanical environment after implantation, where we
expect integration and bone penetration at scaffold. Also,
tantalum has higher friction ratio with bone, in comparison with
other porous materials. This factor increases the initial
stability after implantation. The preparation method of porous
tantalum and the mechanical properties mentioned above, allow
its formation in a variety of simple or complicated shapes,
sometimes even individual patterns, either as coating surfaces,
or as a structural bulky material. As a conclusion, in
comparison with the conventional metallic materials used in
orthopaedic surgery, porous tantalum has a significantly greater
rate of porous texture, lower rigity -similar to cancellous
bone- and higher friction ratio, which is important in the
initial stability after implantation. In comparison with bone
grafts, it has similar geometry, more predictable quality and
mechanical properties, which are constant and not downgraded
after material’s implantation.

Fig 1.
Porous tantalum’s characteristic duodenofundament structure.
In vivo experimental data
Bobyn et al22 studied biological processes at
bone-porous tantalum interface in skeletal mature dogs, after
implantation of a tantalum rod at the femoral cortical bone. The
progress in bone penetration was recorded at 4, 16, and 52
weeks, after excision of the area of implantation and expression
of the bone penetration as the rate of available pores that have
been filled with new bone. Two different sizes of pores were
studied, big (650μm)
and small (430μm)
diameter and the rates of filling at 4-16-52 weeks were: at 4
weeks 52,9% / 41,5%, at 16 weeks 69,2% / 63,1% and at 52 weeks
70,6% / 79,7% respectively. These differences are not important
in clinical practice. A material with smaller pore’s diameter is
preferable, because it is mechanically more stable due to its
thicker scaffold rods. New bone formation at porous tantalum -
implant interface followed fixed pattern: at 2 weeks it appeared
intramedullary and at the limits of the cortical drilled hole.
New bone at scaffold rods was observed in small amounts at 2
weeks, with small increase at 3 weeks. However, at 4 weeks the
new bone’s penetration at porous tantalum was a constant finding
in a big rate of surface and very often at the whole sectioning
diameter. At 16 and 52 weeks bone penetration was dense and
extensive, while the new bone in the scaffold and surrounding
cortical bone had histological findings of bone remodelling.
Haver’s system formation was present in the first case and
increased blood flow and porous texture in the second. At 4
weeks, the mean mechanical power of fixation was 18,5Mpa
(shearing resistance), which is obviously greater in comparison
with other studies of similar protocol involving other metallic
porous materials (1,2-13,1 Mpa). This difference is due to the
higher rate of porous texture, which results, for the same
volume of metallic porous scaffold and the same rate of
available empty space, in a greater amount of bone penetration
(in absolute prices). This fact theoretically leads to higher
mechanical tolerance in a shorter period of time. The same group
studied the porous tantalum’s behavior in a completely
functional, normally loaded model of total hip replacement in
dogs19. The material was used as a metallic shell in
a hemispherical acetabular component where the polyethylene was
integrated in the shell with a special procedure (compression
molding) in a depth of 1 mm. In this way the distal 2mm of the
porous tantalum’s diameter were left available for bone
penetration. The implant was removed after 26 weeks, en block
with the surrounding acetabular bone. After sectioning in
frontal level, in pieces of 2mm thickness, which were separated
in 5 consecutive zones with equal angle, radiological
examination revealed stable adhesion in the circumference (zone
A and E) where the acetabular bone had greater radiological
density. In a line with the dome of the hemispherical component
(zone C), a radiological lucent sign appeared, until 3mm, as it
was expected according to the technique of implantation (press
fit, based on the periphery of the implant). However, in most
cases there was a radiologic impression of filling of the lucent
sign with new bone. This impression was proved after
histological examination. The analysis of bone penetration in
electronic microscope was as following: at zones A, E the
greater amount of new bone formation was measured, approximately
25,1%. At these zones, who are in the periphery of the
acetabulum, the contact between component and bone was obviously
more uniform and the bone more thick. The total amount of
filling was 16,8%, and 17,3% if the zone C was excluded. At this
zone, as expected, the bone’s penetration ranged from nothing to
very little. These rates are comparable with those for other
porous materials. However taking into account the absolute bone
mass per volume of material, the porous tantalum is superior,
thanks to the greater amount of porous texture. This is
equivalent to greater mechanical resistance of the interface.
Signs of new bone formation were observed outside of the
implant’s zone and acetabular limits, rarely with histological
continuity with the new bone at zones A and E. This finding is a
strong sign of osteoinductive properties. Histologically the new
formed bone had normal structure, normal cellularity and
vascularity, without cysts or osteoclastic activity. Pores not
occupied from bone were occupied with dense fibrous tissue. This
is desirable because the lack of free pores acts as a barrier to
the synovial and wear products. We must note here, that porous
tantalum’s ability for adhesion to fibrous tissue and
regeneration of the latter has been studied experimentally by
placing it into dog’s subcutaneous tissue23. Older
studies24 showed that fibrous tissue appears
histologically a healing ability in contact to porous structures
with pore’s diameter bigger than 50μ.
In this study a satisfactory penetration of dense, vascular
fibrous tissue was observed, with similar histological and
mechanical resistance features as a normal healing fibrous
tissue. Andreykiv et al25 interpret the bone
penetration in porous tantalum as a procedure proportional to
bone healing. Under the condition of initial mechanical
stability, the initial filling with granuloma tissue is followed
by the procedure of endomembranous ossification. The meaning of
stable mechanical environment has to do with the limitation of
micro-movements under limits which are different, depending on
the interface. This critical limit has not been elucidated about
the porous tantalum and this is why the above scientists take
into account in their studies the number of 20μm,
which is very low, but offers security to accept the results.
The bone in the interface is a source of vessels and osteoblasts.
After implantation, mesenchymal cells migrate from the surface
to the porous tantalum. Mesenchymatic cells differentiate
according to the model of Prendergast et al26
depending on the stimulation from 2 different biophysical
factors, the maximum tension of shearing deformity
γ
and the relevant velocity liquid/solid
ν.
High levels of these stimulate the differentiation of
mesenchymatic cells to fibroblasts, intermediate levels to
chondral cells, while low levels to osteoblasts. The
differentiation to mixed row cells is possibly based on the
above model. So the initial mechanical stability is important
for the penetration from mesenchymatic cells and the
differentiation to bone.
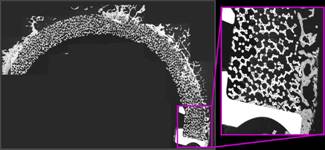
Fig 2.
Deep and extensive bone penetration in an acetabular cup
implanted for 52 weks in a full loading canine model
Histological data from retrieved
implant’s analysis
Histological studies, with electronic microscope, of an
acetabular porous tantalum implant, which was removed after
recurrent hip dislocation two years after surgery27,
revealed bone penetration and bone adhesion in the entire
surface of the implant. (Fig 3) Electronic microscopic study
depicted deep bone penetration, particularly in the periphery of
the shell, without fibrous tissue interposition in bone-tantalum
interface. Histological depiction was compatible with the
radiological depiction of complete implant’s integration.
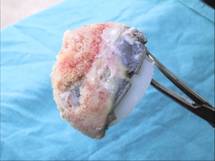
Fig 3.
Retrieved TMT acetabular component. Excision was performed with
the use of a special cutting instrument due to stable bony
fixation.
Clinical data from the use of TMT cups
and other applications
The TMT acetabular component consists of a porous tantalum shell
with an elliptical flat shape geometry and a solid attached
polyethylene after specific preparation (compression molding).
Polyethylene’s penetration into the metallic scaffold is about
1-2mm and available depth left for bone penetration about 2-3mm.
Patients with sufficient bone substrate for peripheral support
of the acetabular component were included in a multicenter
prospective study28, where specific interest was
given in the presence, in the AP pelvis radiograph, of bone gap
and it’s progression. Acetabular-component interface was
separated in 3 contiguous zones 60ο
each, in a variant of De Lee and Charnley zones. Among 574 total
hip arthroplasties, 414 were observed for a minimum of 2 years.
20% presented in the initial radiograph with a “gap” located in
the central zone II, as expected. The width of half
of
these gaps was more than 1mm, ranging 1mm-5mm. At the last
examination (2-5 years) the gap had disappeared in 85% of the
cases, including all of the 5mm gaps, while the same happened
with 10 co-existing osteoarthritic cysts. At arthroplasties
without initial gap, the last examination revealed new lucent
line in 9%, smaller than 1mm, limited in one only zone, with
equal distribution between the three zones. In total, with
radiographic criteria, 412 of 414 arthroplasties were stable,
via bone integration, without migration. The fact that bone gap
filing is taking place with new bone formation and without
acetabular component migration, has been proven by a study of
migration of this acetabular implant with the EBRA software29.
This specific software can estimate the bone gaps with accuracy
rate of 0,1mm and after reduction to scale 1:1,15 based on the
distance between the radiology source and the pelvis, so the
measures are in agreement with the real dimensions. It also
allows the analysis of the radiographs after scanning in a high
analysis electronical scanner and the estimation of possible
implant’s migration in relation to stable parts of the pelvis,
eliminating systematic errors from possible pelvis inclination.
In this study at the equatorial of the implant, “gaps” were
found of 4mm max, at 25 from a total of 180 total hip
arthroplasties during 1998-2001. After 12-24 weeks all the gaps
were restored (Fig 4 and 5), without component migration in any
axis. In specific, implant’s migration was 0,01mm at X axis and
0,02mm at
Ψ
axis, which practically means absence of migration. These hips
were subsequently followed30 for a
time ranged from 8 to 10 years. For the purpose of this study,
151 hips were available for final evaluation. The average
pre-operative total Harris Hip Score was 44.0 ± 13.8 (4 to
86.75), increased at one-year to 95.2 ± 4.8 (81 to 100) (p <
0.05) and remained constant through the latest follow-up at
97.0 ± 6.2, (58.85 to 100) (p < 0.05). The average Oxford Hip
Score improved from a preoperative score of 43.3 ±6.5 to 15.2
±2.3 at one-year postoperatively and 13.9 ±2.3 at the latest
follow up. There was no radiographic evidence of gross
polyethylene wear, progressive radiolucencies, osteolytic
lesions, acetabular fracture or component subsidence. There were
7 (4.5%) postoperative complications all unrelated to the
acetabular component. This study reveals both clinical and
radiographic evidence of well fixed and stable acetabular
components through 8-10 years, thus confirming the initial
hypothesis of increased early stability, safe osteoindegration
and more “physiologic” load transfer of the tantalum acetabular
component. During the last decade porous tantalum has been used
in several orthopaedic applications. Its use in hip arthroplasty
has been extended from primary to revision cases.
Cementless acetabular revision with the tantalum acetabular
shell demonstrated excellent clinical and radiographic results,
even with severe (Paprosky 3A and 3B) acetabular bone defects31,32.
In the area of knee arthroplasty,
porous
tantalum metaphyseal cones33 effectively provide
structural support for the tibial implants and is a good
treatment method for large tibial bone defects during revision
knee replacement. Porous tantalum cone has also been used34
as an “internal plate” for reconstruction of a combined
segmental/cavitary defect of the proximal tibia in difficult
cases sush as Charcot Arthropathy.
Support of femoral head35,36 in avascular necrosis-
Ficat stages I and II- with porous tantalum rods has shown good
results, comparable with those using vascular fibular autograft.
Other applications include salvage patella replacement37,38,
oncological
implants39 for bone integration and soft tissues
adhesion, carpal-cubitocarpal joint fusion40,
arthrodesis in cervical and lumbar spine41,42,
subchondral replacement scaffolds43 in chondral
lesions repair.
Recent experimental
studies in a model of hip arthroplasty in dogs44 have
shown that combining a tantalum’s shell with an intravenous dose
0,1 mg/kg of biphosphonate zoledronic acid immediately after
surgery, led to a 85% greater bone formation into the porous
material, 6 weeks after implantation. This finding means that
porous tantalum in combination with biphosphonates can increase
the biological fixation into the bone’s interface. This
combination, as proved by recent experimental studies45,
can be performed and with local use of biphosphonate after
physical and chemical connection with hydroxyapatite coated
porous tantalum implant: intramedullary implantation in ulna
bone at a dog showed statistically important greater area of
surface been bone integrated and in total, greater volume of new
bone formation in contact and around the implant.
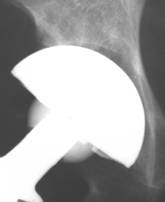 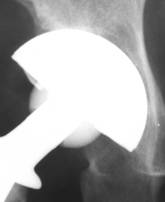
Fig 4.
Polar gap at the initial AP radiograph. The gap was filled with
new bone formation at 24 weeks. Despite the fact that the
periphery of the implant was left uncovered, the arthroplasty
functions well, without signs of loosening at the latest follow
up
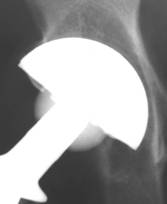 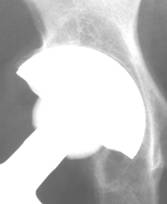
Fig 5.
New bone formation onto uncovered shell. This is a sign of
implant’s osteoinductive properties.
Conclusions:
Porous tantalum is a relatively new biomaterial with unique
mechanical properties. It has a great porosity, up to 80% of its
volume, that favors bone penetration and osteoindegration. Its
elasticity is close to subchondral bone, offering more normal
patterns of load transmission, while at the same time its
adherent nature offers initial stability after implantation;
this ensures a mechanically stable environment which is
important for favorable long term results. The capability of
different shapes and patterns in manufacturing allows the
consideration of porous tantalum in a variety of orthopaedic
surgical applications. For any implant, where we require good
long-lasting functionality, the proof in practice with
continuous observation is essential. This is established by
long-lasting clinical prospective studies, and
laboratory-histological examination of well-functioning implants
which are removed for some reason. Experimental data, however,
and the first to mid-term clinical results, indicate that the
unique physical and chemical properties of porous tantalum
provide new opportunities in construction and application of
orthopaedic implants.
Reference :
-
Cook SD, Barrack RL, Thomas KA, Haddad RJ. Quantitative
analysis of tissue growth into human porous total hip
components. J Arthroplasty 1988;3:249-62.
-
Engh CA, Zettl-Schaffer KF, Kukita Y, et al.
Histological and radiographic assessment of well functioning
porous-coated acetabular components: a human post-mortem
retrieval study. J Bone Joint Surg [Am] 1993;75-A:814-24.
-
Pidhorz LE, Urban RM, Jacobs JJ, Sumner DR, Galante JO. A
quantitative study of bone and soft tissues in cementless
porous-coated acetabular components retrieved at autopsy. J
Arthroplasty 1993;8: 213-25.
-
Black J. Biological performance of tantalum. Clin Mater.
1994;16:167-173
-
Burke GL. The corrosion of metals in tissues and an
introduction to tantalum. Canada Med Ass. J. 1940;43:125-128
-
ASTM. Standard specification for unalloyed tantalum for
surgical implant applications (ASTM F 560-98). In: Annual Book
of ASTM Standards, ASTM, 1998;13.01:63-65
-
Johnson PF, Bernstein JJ, Hunter G, Dawson WW, Hench LL.
An in vitro and in vivo analysis of anodized tantalum
capacitive electrodes: corrosion response, physiology and
histology. J Biomed Mater Res 1977;11:637-56.
-
Pudenz RH. The repair of cranial defects with tantalum: an
experimental study. J Amer Med Assoc 1943;121:478-81.
-
Brown MA, Carden JA, Coleman RE, McKinney R Jr, Spicer LD.
Magnetic field effects on surgical ligation clips. Magn Reson
Imaging 1987;5:443-53.
-
Plenk
H Jr, Pfluger G, Schider S, Bohler N, Grundschober F. The
current status of uncemented tantalum and niobium femoral
endoprostheses. In: Morscher E, ed. The cementless fixation of
hip endoprostheses. Berlin: Springer-Verlag, 1984:174-7.
-
Spurling RG. The use of tantalum wire and foil in the repair
of peripheral nerves. Surg Clin North Am 1943;23:1491-504.
-
Aronson AS, Jonsson N, Alberius P. Tantalum markers in
radiography: an assessment of tissue reactions. Skeletal
Radiol 1985;14: 207-11.
-
Grundschober F, Kellner G, Eschberger J, Plenk H Jr.
Long-term osseous anchorage of endosseous dental implants made
of tantalum and titanium. In: Winter GB, Gibbons DF, Plenk H
Jr, eds. Biomaterials 1980. Chichester: John Wiley & Sons,
1982:365-70.
-
Kokubo T.
, Kim HM et al Metallic materials stimulating bone formation
Med J Malaysia. 2004 May;59 Suppl B:91-2.
-
Findlay DM,
Welldon K,
Atkins GJ,
Howie DW,
Zannettino AC,
Bobyn D
The proliferation and phenotypic expression of human
osteoblasts on tantalum metal. Biomaterials. 2004
May;25(12):2215-27
-
Welldon KJ, Atkins GJ,Howie DW, Findlay DM.Primary human
osteoblasts grow into porous tantalum and maintain an
osteoblastic phenotype. J Biomed Mater Res A. 2008 Mar
1;84(3):691-701
-
Stackpool GJ, Kay AB, Morton P, et al. Bone ingrowth
characteristics of porous tantalum: a new material for
orthopaedic implants. Trans Combined ORS, 1995:45
-
Cohen R. A porous tantalum trabecular metal: basic science –
review paper. The Am J of Orth 2002 Apr;31(4):216-7.
-
Bobyn JD, Toh K-K, Hacking SA, et al. the tissue response to
porous tantalum acetabular cups. A canine model. J Arthr.
1999;14:347-354
-
Kieswetter K, Schwartz Z, Hummert TW, et al.
Surface roughness modulates the local production of growth
factors and cytokines by osteoblast-like MG-63 cells.
J Biomed Mater Res 1996;32:55-63.
-
Krygier JJ, Bobyn JD, Poggie RA, Cohen RC. Mechanical
characterisation of a new porous tantalum biomaterial for
orthopaedic reconstruction.
SIROT, Sydney, 1999.
-
Bobyn JD, G. J. Stackpool, S. A. Hacking, M. Tanzer, J. J.
Krygier . Characteristics of bone ingrowth and interface
mechanics of a new porous tantalum biomaterial JBone Joint
Surg (Br) 1999; 81-B: 907-914.
-
Hacking SA,
Bobyn JD,
Toh K,
Tanzer M,
Krygier JJ.
Fibrous tissue ingrowth and attachment to porous tantalum. J
Biomed Mater Res. 2000 Dec 15;52(4):631-8.
-
Gottsauner – Wolf F, Egger EL, Chao EY, et al. Tendons
attached to prostheses by tendon – bone block fixation. An
experimental study in dogs. J Orth Res 1994;12:814-821
-
Andreykiv A, Prendergast PJ, Kuelen F, Swieszofsky W, Rozing
P. Bone ingrowth simulation for a concept glenoid component
design. J Biomech. 38(2005) 1023-1033
-
Prendergast PJ, Huiskes R, Soballe K. Biophysical stimuli on
cells during tissue differentiation at implant interfaces. J
Biomech. 1997 30(6), 539-548
-
Bobyn JD (Jo Miller Laboratory, Montreal, Canada), Case:
Michelinakis E
-
Gruen TA, Poggie RA, et al. Radiographic Evaluation of a
Monoblock Acetabular Component.
A Multicenter Study with 2- to 5-Year Results. J Arthr 2005
20(3): 369-378
-
Kostakos AT,
Macheras GA,
Frangakis CE,
Stafilas KS,
Baltas
D,
Xenakis
TA, Migration of the Trabecular Metal Monoblock
Acetabular Cup System A 2-year follow-up using the
Ein-Bild-Röntgen-Analyse method.
J
Arthroplasty. Acc. 2008 Dec, ahead of print
-
Macheras G, Kateros K, Kostakos A, Koutsostathis S, Danomaras
D, Papagelopoulos PJ, Eight- to Ten-Year Clinical and
Radiographic Outcome of a Porous Tantalum Monoblock Acetabular
Component. J Arthroplasty.
2009 Aug;24(5):705-9
-
Kim WY, Greidanus NV, Duncan CP, Masri BA, Garbuz DS. Porous
tantalum uncemented acetabular shells in revision total hip
replacement: two to four year clinical and radiographic
results. Hip Int. 2008 Jan-Mar: 18(1):17-22
-
Weeden
SH,
Schmidt
RH. The use of tantalum porous metal implants for
Paprosky 3A and 3B defects. J Arthroplasty.
2007 Sep;22(6 Suppl 2):151-5.
-
Meneghini RM,
Lewallen DG,
Hanssen
AD. Use of porous tantalum metaphyseal cones for
severe tibial bone loss during revision total knee
replacement. J Bone Joint Surg Am. 2008
Jan;90(1):78-84.
-
Troyer J. Levine BR.Proximal tibia reconstruction with a
porous tantalum cone in a patient with Charcot arthropathy.
Οrthopedics 2009 May: 32(5):358
-
Veillette CJ,
Mehdian
H,
Schemitsch EH,
McKee
MD. Survivorship analysis and radiographic outcome
following tantalum rod insertion for osteonecrosis of the
femoral head. J Bone Joint Surg Am. 2006
Nov;88 Suppl 3:48-55.
-
Tsao AK,
Roberson JR,
Christie MJ,
Dore DD,
Heck DA,
Robertson DD,
Poggie RA.
Biomechanical and clinical evaluations of a porous tantalum
implant for the treatment of early-stage osteonecrosis. J Bone
Joint Surg Am. 2005;87 Suppl 2:22-7.
-
Nasser S,
Poggie RA
Revision and salvage patellar arthroplasty using a porous
tantalum implantJ Arthroplasty. 2004 Aug;19(5):562-72.
-
Ries MD, Cabalo A., Bozic KJ, Anderson M.Porous tantalum
patellar augmentation: the importance of residual bone stock.
Clin Orthop Relat Res 2006 Nov :452:166-70
-
Chalkin B,
Minter J.
Limb salvage and abductor reattachment using a custom
prosthesis with porous tantalum components. J Arthroplasty.
2005 Jan;20(1):127-30.
-
Adams JE,
Zobitz ME,
Reach JS Jr,
An KN,
Lewallen DG,
Steinmann SP
Canine carpal joint fusion: a model for four-corner
arthrodesis using a porous tantalum implant. J Hand Surg [Am].
2005 Nov;30(6):1128-35.
-
Zou X,
Li H,
Teng X,
Xue Q,
Egund N,
Lind M,
Bunger C
Pedicle screw fixation enhances anterior lumbar interbody
fusion with porous tantalum cages: an experimental study in
pigs. Spine. 2005 Jul 15;30(14):E392-9.
-
Wigfield C,
Robertson J,
Gill S,
Nelson R.Clinical
experience with porous tantalum cervical interbody implants in
a prospective randomized controlled trial. Br J Neurosurg.
2003 Oct;17(5):418-25.
-
Mardones RM,
Reinholz GG,
Fitzsimmons JS,
Zobitz ME,
An KN,
Lewallen DG,
Yaszemski MJ,
O'Driscoll SW.
Development of a biologic prosthetic composite for cartilage
repair. Tissue Eng. 2005 Sep-Oct;11(9-10):1368-78.
-
Bobyn JD,
Hacking SA,
Krygier JJ,
Harvey EJ,
Little DG,
Tanzer M.
Zoledronic acid causes enhancement of bone growth into porous
implants 2005Mar;87(3):416-20.
-
Tanzer M,
Karabasz D,
Krygier JJ,
Cohen R,
Bobyn JD.The
Otto Aufranc Award: bone augmentation around and within porous
implants by local bisphosphonate elution. Clin Orthop Relat
Res. 2005 Dec;441:30-9.
|









