|
Abstract:
Fibroblast
growth factor 23 (FGF23), a protein capable of inhibiting renal
tubular epithelial phosphate transport, is thought to be the
underlying mechanism in tumor-induced- osteomalacia. In this
report, we present a case of tumor-induced- osteomalacia
associated with an oral soft tissue tumor that caused
overexpression of FGF23.
A 35-year-old man suffered from bone pain for 2 years and he
presented the difficulty in walking due to severe bone pain and
muscle weakness in his lower limbs. Radiography identified old
fractures and severe bone atrophy at multiple bones and bone
scintigraphy revealed abnormal tracer uptake. Laboratory
findings showed that the serum phosphate level was very low (0.8
mg/dl), and FGF23 serum level was elevated (128.7 pg/ml). We
diagnosed the patient as FGF 23-producing tumor-induced
hypophosphatemic osteomalacia a. About 2 years after the start
of our follow-up, a small soft tumor was found behind of the
lower lip. After resection of the tumor, the serum phosphate and
FGF23 levels rapidly normalized and the severe bone pain
markedly improved. Furthermore, the tissue was stained positive
using anti-human FGF23 antibody. In this report, we presented
the rare case that FGF 23–producing tumor within the oral cavity
inducing osteomalacia with severe clinical symptom including
bone pain, multiple fragility fractures, muscle weakness and
gait disturbance.
J.Orthopaedics 2009;6(4)e11
Keywords:
fibroblast
growth factor (FGF 23); tumor-induced osteomalacia; oral soft
tumor; bone pain; phosphate
Introduction:
Osteomalacia is a metabolic bone disorder resulting from
inadequate mineralization of osteoid in mature bone. One of the
most unusual types of osteomalacia is tumor-induced osteomalacia,
which is characterized clinically by bone pain, fractures,
muscle weakness, gait disturbance, renal phosphate wasting,
hypophosphatemia, decreased serum 1, 25-dihydroxyvitamin D
levels, and resistance to vitamin D supplementation (1). Recent
reports have indicated that fibroblast growth factor 23 (FGF23)
is a protein capable of inhibiting renal tubular epithelial
phosphate transport (2), which is thought to be the underlying
mechanism in tumor-induced- osteomalacia2. FGF23 has been
identified as a member of the FGF family and shown to have an
important role in phosphate homeostasis (3) Most of the tumors
causing osteomalacia are benign and occur in the lower and upper
extremities; however, the tumors are frequently very small,
making discovery difficult (4, 5).
In this report, we present a case of tumor-induced- osteomalacia
associated with an oral soft tissue tumor that caused
overexpression of FGF 23, and the rapid normalization of FGF23
levels following removal of the tumor. Subsequently, the
normalization of biochemical parameters of mineral metabolism
correlated with the improvement in severe bone pain and
activities of daily living (ADL).
Case Report:
A
35-year-old man had experienced bone pain in ribs and right hip
for 2 years. At 6 months after the onset of pain, he underwent a
medical examination to establish the cause of the bone pain in
another hospital. However, the origin of the symptoms could not
be found, and the patient was followed up with non-steroidal
anti-inflammatory drug treatment for over 1.5 years. The
symptoms gradually worsened and he presented the difficulty in
walking due to severe bone pain and muscle weakness in his lower
limbs. The patient was referred to our hospital for further
examination because of the unknown origin of the symptoms.
The patient
complained of a severe diffuse bone pain all over his body. He
was wheelchair bound due to significant pain in hips and knees
with a visual analogue scale (VAS) of 8.4, and muscle weakness
of lower limbs. He did not have a significant family history of
bone diseases or any history of other diseases. Previously he
had been healthy and worked as a truck driver with a high level
of physical activity. His height, weight, and body mass index
was 159.1 cm, 52.6 kg, and 20.8 kg/m2, respectively.
The muscle strength of the lower limbs was found to be grade 3
on the basis of manual muscle testing. We found no other
abnormalities on physical examination. Radiography identified
multiple old fractures and severe bone atrophy at spine, ribs,
pubis, ischium and lower limbs (data not shown). Bone mineral
density (BMD) was markedly decreased at the lumbar spine (L2-L4;
0.512 g/cm2, T score -3.8) and femoral neck (0.478
g/cm2, T-score -3.6) (QDR4500; Hologic, Waltham, MA,
USA). Bone scintigraphy with 99mTc-methylene
diphosphonate revealed abnormal tracer uptake in multiple bones
including the spine, ribs, pelvis and femurs, suggesting
clinical fractures (Fig. 1). Laboratory findings showed that the
serum phosphate (IP, reference range 2.5-4.5 mg/dl) level was
very low (0.8 mg/dl), and serum total alkaline phosphatase (ALP,
reference range 110-370 IU/l) and bone specific alkaline
phosphatase (BAP, reference range 13-33.9 U/l) levels were
markedly increased at 1878 IU/l and 312 U/l, respectively. The
urinary N-terminal telopeptide of type I collagen (uNTX,
reference range, < 66.2 nmol
bone collagen equivalent / mmol·creatinine
[nMBCE/mM·Cr]) level was 43.7
nMBCE/mM·Cr, and the circulating 1,25-dihydroxyvitamin D (1,25(OH)2D,
reference range, 20-60) level was very low (8 pg/ml) despite the
hypophosphatemia. Several serum tumor markers, such as CEA, AFP,
CA19-9 and PSA, were within reference ranges. Other laboratory
data were also within reference ranges. Thus, we diagnosed the
patient as hypophosphatemic osteomalacia, which was confirmed by
an iliac bone biopsy. Furthermore, considering that he had been
healthy for 33 years prior to the onset of his disease and that
the symptoms had worsened during the 2-year- follow-up,
tumor-induced- osteomalacia was considered to be the most
probable cause of this patient.
However, no
significant abnormalities apart from skeletal organs were
identified using computed tomography, ultrasonography, 201Tl
scintigraphy, and endoscopy for the digestive organs.
Furthermore, a positron emission tomography (PET) scan failed to
reveal any abnormalities in any part of the body. On the basis
of a recent report that venous sampling for FGF23 confirms
preoperative diagnosis of tumor-induced- osteomalacia (6), we
measured FGF23 concentration by ELISA (Kainos, Japan) in
subcutaneous venous samples (reference range, 10-50 pg/ml) taken
from 6 points in the upper and lower extremities. The FGF23
serum level was elevated in samples from all 6 points, including
the bilateral hands (right 181.4, left, 195.5 pg/ml), femurs
(right 181.4, left 222.3 pg/ml) and lower legs (right 189.1,
left 183.6 pg/ml); however, there were no differences among
samples. From these results, we diagnosed the patient as FGF
23-producing tumor-induced hypophosphatemic osteomalacia
although no causative tumor was identified. Treatment with
alfacalcidol (3.0 µg / day) and sodium acid phosphate (500mg of
elemental phosphorus three times daily) was initiated on the
basis of the diagnosis. Subsequently, his pain slightly
improved, but the patient still suffered from multiple bone pain
and presented with the difficulty in walking without aid.
About 2
years after the start of our follow-up, the patient became aware
of a small soft tumor behind of the lower lip. Six months later,
the tumor size gradually increased until it was 23mm × 14mm ×
10mm in size (Fig. 2A) so that the patient underwent resection
of the tumor. The pathologic diagnosis of the tumor was
consistent with fibroma, and revealed a fibroblastic
proliferation with fibrous connective tissues and reactive bone
formation without any evidence of malignancy (Fig. 2B).
Furthermore, the tissue was stained positive using polyclonal
rabbit anti-human FGF23 antibody (FGF23 (FL251)) (1:500
dilution; Santa Cruz Biotechnology, CA, USA) (Fig 2C).
At 5 hours
post-surgery, the serum FGF 23 level rapidly decreased to a
reference range, and thereafter remained under 10 pg/ml (Fig.
3A). The serum phosphate level rapidly increased and remained
within the reference range for more than 9 months post-surgery
(fig. 3B). ALP initially rose following tumor removal and then
gradually declined. Normalization of the ALP was not achieved
until 6 months post-surgery (Fig. 3C). The severe bone pain of
the patient gradually decreased although moderate pain remained
post-operatively (Fig. 3D). At 6 months post-operatively, the
patient was able to walk without any supports and had resumed
low-level physical activity. General health was assessed with
use of the Short Form-36 (SF-36) (7, 8) and post-operative SF-36
scores were improved compared with pre-operative scores (Fig.
4).
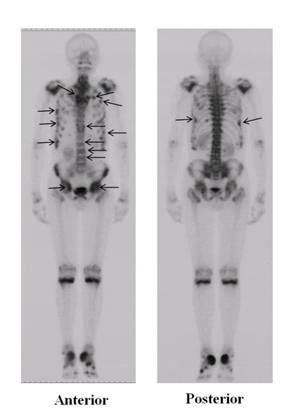
Figure 1
Bone scintigraphy of the patient
Bone
scintigraphy with 99mTc-methylene diphosphonate
revealed abnormal tracer uptake in multiple bones including the
spine, ribs, pelvis and femurs (arrows).
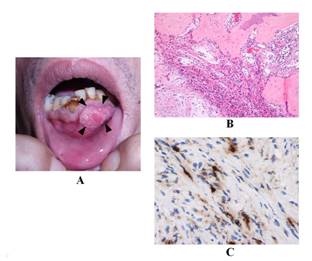
Figure 2
FGF23 producing tumor within the oral cavity
The soft
tumor was located behind the lower lip (tumor size was 23mm ×
14mm × 10mm) (A, arrow heads). Pathologic diagnosis of the tumor
was consistent with fibroma, and revealed a fibroblastic
proliferation with fibrous connective tissues and reactive bone
formation without evidence of malignancy (B, Hematoxilin-Eosin
stain). The tissue was stained positive using polyclonal rabbit
anti-human FGF23 antibody (C, single immunolabeling (peroxidase
and DAB).
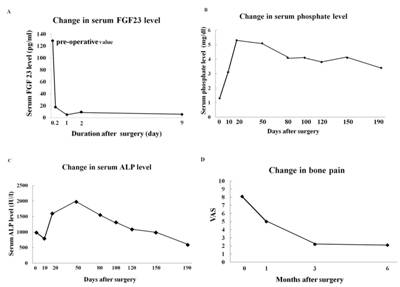
Figure 3
At 5 hours
post-surgery, the serum level of FGF23 rapidly decreased from
128.7 pg/ml to 17.6 pg/ml, and thereafter remained under 10
pg/ml (A). The serum phosphate level rapidly increased to 3.1
mg/dl and remained within the reference range (2.5 – 4.5 mg/dl)
for more than 9 months post-surgery (B). ALP initially rose
following the tumor removal and then gradually declined.
Normalization of the ALP (110 – 370) was not achieved until 6
months post-surgery (ALP, 585 IU/l) (C). The severe bone pain
(VAS 8.1) gradually decreased, although moderate pain (VAS 2.1)
remained postoperatively (D).
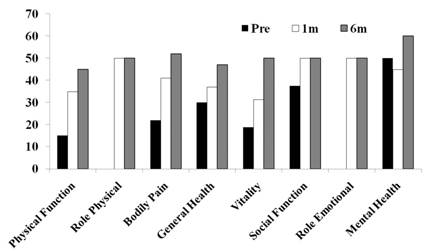
Figure
4
Pre- and 1-
and 6-month post-operative SF-36 scores
The
scores for the eight subscales of the SF-36 (Physical Function,
Role Physical, Bodily pain, General Health, Vitality, Social
Function, Role Emotion and Mental Health) were measured prior to
the surgery (black bars) and at 1 month (white bars) and 6
months (striped bars) post-surgery
Discussion :
Tumor-induced osteomalacia is an acquired, paraneoplastic
syndrome that is characterized by hypophosphatemia due to renal
phosphate wasting, osteomalacia, bone pain, proximal muscle
weakness, fractures and functional disability (1). The disease
shares similar of clinical symptoms with X-linked
hypophosphatemia (XLH), autosomal dominant hypophosphatemic
rickets (ADHR), and autosomal recessive hypophosphatemic rickets
(ARHR) (9-12). In this case, the patient had no family history
so that we preoperatively suspected tumor-induced- osteomalacia
rather than genetic diseases such as XLH, ADHR and ARHR.
Interestingly, the site of the tumor was within the oral cavity
despite the fact that most tumors responsible for tumor-induced-
osteomalacia is localized in the lower and upper limbs. To the
best of our knowledge, there have been few previous reports of
FGF 23–producing tumors within the oral cavity inducing
osteomalacia (1, 4, 5).FGF 23 was recently identified as a
causative humoral factor for tumor-induced- osteomalacia, and is
known to reduce serum phosphate level by inhibiting proximal
tubular phosphate reabsorption and decreasing serum 1,25(OH)2D
levels (2, 13). Endo et al. (14) proposed a set of diagnostic
criteria based on the serum phosphate and FGF 23 levels as
follows; in adults, a serum phosphate level of less than 2.5
mg/dl and a FGF 23 level of more than 30 pg/ml by intact FGF 23
assay indicate the presence of diseases caused by excess FGF 23,
such as tumor-induced- osteomalacia and XLH. In our case, the
data from the patient met these criteria, with the serum FGF23
level, in particular, greater than 180 pg/ml.
Measurement
of serum FGF 23 level in samples obtained from peripheral veins
is useful for the diagnosis and post-operative monitoring of
patients with tumor-induced- osteomalacia (6). However, we could
not locate the responsible tumor in the oral cavity because the
FGF23 levels were elevated in the samples from both the upper
and lower limbs, and the patient rejected further invasive
examination, such as sample collection from all major veins
through a catheter inserted through the femoral vein (6).
Because the tumor was found in the oral cavity, it is reasonable
that FGF23 levels from extremities showed no difference. It is
possible that FGF23 was higher if FGF23 levels in jugular veins
could be measured.
Various
types of mesenchymal tumors associated with tumor-induced-
osteomalacia have been reported, including hemangiopericytoma,
hemangioma, giant cell tumor and osteoblastoma (4, 5). Weidner
and Santa Cruz (4) coined the term “phosphaturic mesenchymal
tumor, mixed connective tissue variant” (PMTMCT) to describe
these unique lesions, characterized by a distinctive admixture
of spindled cells, osteoclast-like giant cells, microcysts,
prominent blood vessels, cartilage-like matrix, and metaplastic
bone. Although the pathologic diagnosis in our case was fibroma,
the histological features could be included into a spectrum of
PMTMCT described as the most frequent histology associated with
tumor-induced- osteomalacia (4, 5).
In this
report, we described a severe case of tumor-induced-
osteomalacia associated with a histologically diagnosed fibroma
that secreted high levels of FGF23. In most orthopaedic clinics,
it is sometimes difficult to distinguish severe osteomalacia
from osteoporosis as the clinical symptoms of both, including
low BMD, bone pain and multiple fragility fractures are similar.
In fact, this case was also diagnosed as a severe osteoporosis
before consultation at our hospital. We believe that it is
important to consider osteomalacia associated with FGF
23-producing- tumor in the differential diagnosis of patients
presenting with the sudden onset of low BMD, bone pain,
fragility fractures and hypophosphatemia. The informed consent
for the publication of data from the case was received from the
patient and the family.
Reference :
-
Schapira
D, Ben Izhak O, Nachtigal A, Burstein A, Shalom RB, Shagrawi
I, et al. Tumor-induced osteomalacia. Seminars in Arthritis
Rheumatism 1995; 25:35-46.
-
Shimada
T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al.
Cloning and characterization of FGF23 as a causative factor of
tumor-induced osteomalacia. Proceedings of the National
Academy of Sciences 2001; 98:6500-6505.
-
Fukumoto
S, Yamashita T. FGF23 is a hormone-regulating phosphate
metabolism-Unique biological characteristics of FGF23. Bone
2007; 40:1190-1195.
-
Weidner
N, Santa Cruz D. Phosphaturic mesenchymal tumors: a
polymorphous group causing osteomalacia or rickets. Cancer
1987; 59:1442-1454.
-
Folpe AL,
Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY,
et al. Most osteomalacia-associated mesenchymal tumors are a
single histopathologic entity: an analysis of 32 cases and a
comprehensive review of the literature. American Journal of
Surgical Pathology 2004; 28: 1-30.
-
Takeuchi Y, Suzuki H,
Ogura S, Imai R, Yamazaki Y, Yamashita T, et al. Venous
Sampling for fibroblast growth factor-23 confirms preoperative
diagnosis of tumor-induced osteomalacia. Journal of Clinical
Endocrinology and Metabolism 2004; 89: 3979-3982.
-
Fukuhara S, Bito S,
Green J, Hsiao A, Kurokawa K. Translation, adaptation, and
validation of the SF-36 Health Survey for use in Japan.
Journal Clinical Epidmiology 1998; 51: 1037-1044.
-
Fukuhara S, Ware JE,
Kosinski M, Wada S, Gandek B. Psychometric and clinical tests
of validity of the Japanese SF-36 Health Survey. Journal
Clinical Epidmiology 1998; 51: 1045-1053.
-
ADHR
Consortium. Autosomal dominant hypophosphataemic rickets is
associated with mutations in FGF23. Nature Genetics
2000; 26:345-348.
-
Hyp-Consortium. A
gene (PEX) with homologies to endopeptidases is mutated in
patients with X-linked hypophosphatemic rickets. Nature
Genetics 1995; 11: 130-136.
-
Lorenz-Depiereux
B, Bastepe M, Benet-Pagčs A, Amyere M, Wagenstaller J,
Müller-Barth U, et al. DMP1 mutations in autosomal recessive
hypophosphatemia implicate a bone matrix protein in the
regulation of phosphate homeostasis. Nature Genetics
2006; 38: 1248-1250.
-
Feng JQ,
Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1
causes rickets and osteomalacia and identifies a role for
osteocytes in mineral metabolism. Nature Genetics 2006;
38: 1310-1315.
-
Shimada
T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al.
FGF-23 is a potent regulator of Vitamin D metabolism and
phosphate homeostasis. Journal of Bone and Mineral Research
2004; 19:429-435.
-
Endo I,
Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, et al.
Clinical usefulness of measurement of fibroblast growth factor
23 (FGF23) in hypophosphatemic patients: Proposal of
diagnostic criteria using FGF23 measurement. Bone 2008; 42:1235-1239.
|






