|
Abstract:
The
risk of disease transmission from human musculoskeletal
allograft tissue distributed by tissue banks is a subject of
ongoing concern. Efforts
to minimize disease transmission have generally included donor
screening, bioburden assessment, aseptic handling, chemical
washes, antibiotics, and gamma irradiation.
Without adequate sterilization, however, viral and
bacterial contamination of allograft tissue remains a
significant problem.
Achieving terminal sterilization to a sterility assurance
level of 10-6 (SAL6; the standard for medical
devices) frequently compromises biomechanical properties in the
allograft. Here
we report that SAL6 sterilization of human allografts can be
achieved using supercritical CO2 with a peracetic
acid-based additive.
The biomechanical properties of allografts sterilized
with supercritical CO2 are superior to those
sterilized by traditional gamma irradiation. These findings
suggest that supercritical CO2 sterilization of human
allograft tissue will increase the safety of allograft tissues
while maintaining biomechanical properties.
J.Orthopaedics 2009;6(2)e9
Keywords:
sterilization;
supercritical carbon dioxide; allograft tissue; bone
Introduction:
The importance of
allograft tissue in the treatment of musculoskeletal disorders
has grown dramatically since 1990. Such disorders are the most
frequently identified impairments of physical health in the
United States (US). It has been estimated that over 36 million
Americans suffer from musculoskeletal conditions that limit
their ability to function, with costs to society exceeding one
billion dollars annually (1).
Allograft tissue is
widely used by transplant surgeons for orthopedic (joint
replacement), trauma, and cancer (surgical reconstruction)
procedures (2), (3), (4), (5). In the US, tissue bank
distribution of such allografts has increased from 350,000
specimens in 1990 to over 1.6 million in 2005 (1), (6). The
need for safe and effective allografts continues to grow as
allogenic transplantation has become the clinical therapeutic
strategy of choice to combat musculoskeletal disorders.
US tissue banks that
supply allografts from cadavers have traditionally operated with
relatively little regulatory oversight. In the past, bacterial
and viral infections derived from transplanted musculoskeletal
tissues have been rare events. However, the potential for
disease transmission remains a major concern for clinicians and
patients (7). Now, with demand growing for allografts, the US
Food and Drug Administration (FDA) has introduced requirements
for the manufacture of allograft tissue [21 CFR parts 1270 and
1271].
Despite more stringent
regulations, tissue banks continue to utilize aseptic processing
strategies, which may increase the potential risk to patients
from non-sterile allografts. Current allograft processing
techniques often involve several disinfectant steps following
aseptic donor harvest, including soaking in disinfectants and
exposure to a sterilization step (8), (4), (9). Allograft
tissue samples processed by such methods have generally failed
to achieve sterility assurance levels of 10-6 (SAL6) as required
for the sterilization of medical devices (3), (4), (8), (10).
Moreover, preserving the osteogenic and biomechanical properties
of structurally complex allograft tissue has proven to be
challenging.
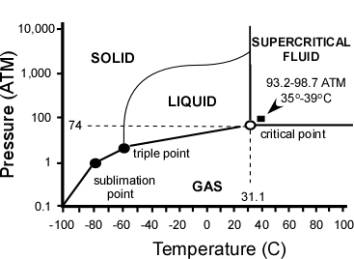
Figure
1 – Phase Diagram of CO2.
Carbon Dioxide has four distinct phases; the standard solid,
liquid and gas as well as the unique supercritical phase.
We have developed a
method for sterilizing musculoskeletal allografts using
supercritical carbon dioxide (SCCO2) [US patent 7,108,832] that
involves the use of low temperature, low pressure and
proprietary nontoxic sterilization additive. Carbon dioxide
has a unique critical point, defined by pressure (Pc=1,099 psi)
and temperature (Tc=31.1˚C) at which the liquid and vapor phases
become indistinguishable (Figure 1). With its low surface
tension, liquid-like density and gas-like diffusivity, SCCO2 is
also ideal for temperature sensitive materials.
Previous research has
shown that proteins and other macromolecules are unreactive
towards SCCO2 (11). SCCO2 successfully inactivates viruses and
produces minimal inflammatory reaction and satisfactory
integration when used in conjunction with hydrogen peroxide on
sheep bone allografts (12), (13). Here we describe a novel
method to achieve SAL6 using musculoskeletal allograft tissue
that is terminally sterilized in double Tyvek® packaging.
Moreover, we demonstrate that the sterilization process does not
compromise the biomechanical properties of the bone allograft.
The method achieves terminal sterilization while avoiding the
damage caused by gamma or other processing methods. Our
experiments further indicate that sterilization by SCCO2
represents a viable solution to the pressing need to terminally
sterilize musculoskeletal allograft tissue.
Materials
and Methods:
Allograft tissue samples
Bone-tendon-bone (BTB)
and large tendon (Achilles and Tibialis; designated as research
tissue) were provided by Community Tissue Services (CTS)
(Dayton, OH). Biomechanical studies utilized nine sets of
trisected BTB samples from nine different donors (three BTBs
harvested per donor).
Cortical rings (femur
and tibia) 15mm in height were also provided by CTS. Femoral
cortical bone struts measuring 4mm (W) x 4mm (H) x 30mm (L) were
used in biomechanical studies. Twelve groups of three struts
per group were procured from a single donor at the same femoral
location and with the same orientation.
Allograft tissue
samples used in sterilization protocols were processed either by
Allowash™ or rinsed only with normal saline solution (0.9g NaCl).
Tissue designated as “Allowashed” was processed using Allowash™
(LifeNet, VA) at CTS prior to SCCO2 treatment at NovaSterilis.
Tissue designated as “saline rinsed” was rinsed extensively with
sterile saline solution at NovaSterilis prior to SCCO2
treatment.
Sterilization Protocols/ Microbiological Assays
Gamma irradiation for
biomechanical studies was performed by Sterigenics (Westerville,
OH) at ambient temperature using a dose of 15-25 kGy.
SCCO2 sterilization was
performed in-house using the 20 L Nova2200™ instrument.
Sterilization runs were conducted using NovaKill™ additive (peracetic
acid based additive; 16 mL per run). Additive was pipetted onto
a 1 ½” x 7 ⅞” cellulose pad, which was then secured in the
chamber’s lower 1” stainless steel basket using a holder.
Allograft samples terminally sealed in double Tyvek® pouches
were arranged in stainless steel baskets (7” and 5”), which were
stacked in the chamber. The Nova2200™ containing samples and
additive was then charged with CO2 from ambient conditions in
6-9 minutes to a pressure of 1436 ± 70 psi and a temperature of
35 ± 3˚C with constant stirring (680 ± 20 rpm). System
parameters and run times were maintained as specified, following
which the vessel was depressurized over 15-25 minutes.
Sterilization run times
for tendon or bone allografts inoculated with B. atrophaeus
spores were determined conservatively by fraction negative
analysis, following ISOs 11138-1, 14937, and 11737-1.
Allograft samples were inoculated with Bacillus atrophaeus spore
suspensions (>106 CFUs/10μL in aqueous solution) (SGM Biotech,
MT) for fraction negative testing. Inocula (10μL) were allowed
to permeate the grafts at room temperature for 15 minutes. Each
allograft sample was transferred to the appropriately sized
Tyvek® gas-permeable pouch, which was sealed before being
inserted into a second terminally sealed Tyvek® pouch prior to
sterilization treatment.
Allograft samples were
then exposed to SCCO2 for various time intervals. To assay for
microbiological growth following treatment, allograft tissue
samples were aseptically removed from packaging and cultured in
bottles containing tryptic soy casein broth (Bacto) at 35˚C.
Each sample culture was observed daily for turbidity over a
period of 7 days and scored for no growth (0) or growth (+).
Samples scoring negative for growth after 7 days were spiked
with 10-100 CFUs B. atrophaeus to test for bacteriostasis. If
growth was observed in the spiked media, the sterility test was
affirmed and the tissue judged sterile.
The total time or full
cycle of the process corresponds to twice the time calculated to
result in the 6 log reduction as demonstrated by the time to
achieve total kill in microbiological methods. This method is
in compliance with the “overkill” methodology outlined in ISO
11737-1 and as required for SAL6.
Biomechanical Testing
Biomechanical testing
was performed by IMR Test Labs (Lansing, NY). Creep testing was
performed on an Instron Dynamite fatigue tester (Model 8841).
Each BTB allograft was clamped on bone sections using standard
wedge grips. The samples were manually cycled three times from
50N to 80N for preconditioning and loaded to 8N with the width
of thickness of the tendon in the midpoint measured using
digital calipers. To determine creep measurements, the gap
between the substantial bone segments was also measured.
Samples were then
subjected to tension cycles (1 sec) from 50N to 250N in a
sinusoidal waveform, followed by a hold (15 sec) at 50N for a
total of 100 cycles. The final length between the bone sections
was measured at 8N and the creep of the samples calculated as in
Equation 1.
Creep (%) = (final
length – original length)/original length x 100
Equation 1
The samples were loaded
to failure using an Instron Tensile Tester (Model 5584). The
tibia section of the BTB was mounted inside plastic cylinders
using epoxy resin. A 3/8” diameter dowel was inserted between
the bone end and the tendon to prevent tearing. The midpoint of
each tendon was trimmed to 5 mm. The patellar bone section was
placed in standard wedge grips. The reduced section width was
measured and the samples were then pulled at a crosshead speed
of 0.5 inches per minute until a significant load drop was
recorded. From the load deflection data, the peak stress,
elongation at break (based on 1 mm gauge length) and modulus
were calculated.
For tendon
biomechanics, 9 sets of trisected BTBs consisting of 3 BTBs
harvested per donor (27 total samples) were divided into three
groups. SCCO2 sterilization was carried out for 4 hours
(overkill from half cycle 90 min; SAL6). All BTBs were loaded
onto an Instron tensile testing machine and forced to failure at
a rate of 1 inch elongation/minute. Tensile strength was
calculated as the maximal load divided by the cross sectional
area.
For bone biomechanics,
36 bone struts (4mm x 4mm x 30mm) consisting of 3 cortical
struts harvested per donor from the same location and
orientation were divided into 3 groups with 12 struts each (one
per donor). These groups consisted of untreated, traditional
gamma irradiated, and SCCO2 sterilized. SCCO2 sterilization was
carried out for 70 minutes (overkill, strut half cycle 20
minutes). Subsequent to the respective treatments all bone
struts were measured for density, 3 point bending, ash fraction,
and collagen cross-linking.
Bone density
measurements were performed according to ASTM D 792-00. The
struts were subsequently incubated at room temperature in
phosphate-buffered saline (PBS) for 48 hours and kept moist
during the testing. Three point bending was carried out in
accordance with ASTM D 790-03 using an Instron Model 5584
Universal Tester controlled by Instron Merlin software. The
specimens were supported using a 20mm span (L) with a 4.8mm
loading diameter. The load was applied to the center of the
specimen (L/2) at a rate of 1 mm/min. Flexural modulus was
calculated from the slope of the loading curve. Flexural
strength was calculated following ASTM D 790-03 by integrating
the area under the stress-strain curve.
After the bending tests
were completed, the two halves of the failed specimens were
examined to determine mineral content and collagen
cross-linking, respectively. Ash fraction was measured by
ashing the specimens (ash mass/dry mass) (ASTM D 4630-01 with
slight modification). Bone samples were dehydrated by
incubating at 100˚C for 48 hours, weighed, and then incubating
at 800˚C for 24 hours. The remaining ash was weighed and
divided by the dry weight to determine the ash fraction.
Collagen cross-linking
Collagen cross-linking
analysis was performed by Articular Engineering, LLC
(Northbrook, IL). Samples were first powdered using liquid
nitrogen, demineralized using ethylenediaminetetraacetic acid (EDTA),
and papain digested. For each digest, two aliquots (2 mL each)
were hydrolyzed using hydrochloric acid (HCl). Hydroxy-lysyl
pyridinoline and lysyl pyridinoline were separated using
cellulose column chromatography and lyophilized samples were
resuspended in 400 μL of 1% heptafluorobutyric acid in double
distilled H2O (ddH2O). Aliquots (100 μL) were assayed using
high performance liquid chromatography (HPLC).
Calculations
Statistically
significant differences between experimental and control groups
were determined using analysis of variance (ANOVA) and paired
T-testing using Prism graphpad software (Graphpad Software, CA).
Results :
The term “Sterility
Assurance Level” (SAL), is routinely used in relationship to
sterilization and is the probability of one microbial survivor
is one in one million. B. atrophaeus endospores have been
previously shown to be the most resistant microorganism to
sterilize using our SCCO2 technology (11). Inactivation
kinetics of B. atrophaeus was examined by construction of a
survivor curve by plotting the percent survival of B. atrophaeus
as a function of time. Ideally, the survivor curve is linear
over the full range of inactivation. Using the fraction
negative method we show linear inactivation of greater than 106
CFUs B. atrophaeus spores on saline rinsed tendon using SCCO2
sterilization (Figure 2). Similar data was obtained for bone,
but is not shown. Samples of Allowashed™ tendon exposed to SCCO2
for 90 minutes cultured negative for B. atrophaeus growth, while
also passing the bacteriostasis testing. Similar sterilization
of saline-rinsed tissue required 120 minutes. Both times of
inactivation are representative of half cycles. SAL6, full
cycle sterilization times were 180 minutes and 240 minutes,
respectively.
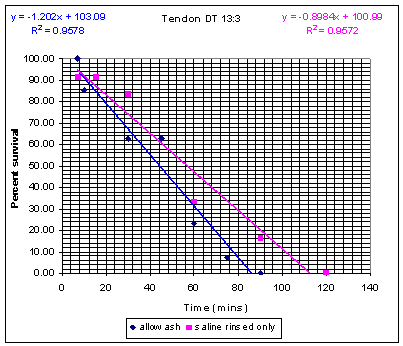
Figure
2. Survival curve for inactivation of Bacillus
atrophaeus endospores inoculated saline rinsed tendon or
allowashed preprocessed tendon. Survival
curve for inactivation of Bacillus
atrophaeus endospores as a function of time.
Saline rinsed tendon (square) or allowashed tendon (diamond) was
inoculated with greater than 106 CFUs and subjected to
sterilization using SCCO2 and NovaKill additive at different
times. The results were calculated as positive
growth/total growth as a function of time.
Biomechanical
Figure
3 depicts differences in the biomechanical properties of
differently processed BTBs.
In samples exposed to gamma radiation, the data reveal
variation in elongation and creep for several donors.
Since elongation is a factor in creep, these observations
were unsurprising.
Although some individual donor differences in the
treatment groups were apparent, ANOVA statistical analysis
reveals no significant difference in BTB biomechanics between
the groups (Table 1).
|
|
Group
|
Mean
|
SE
|
|
|
Gamma
|
3.8
|
1.096
|
|
Creep
(%)
|
Supercritical
CO2
|
2.36
|
0.33
|
|
p
= 0.569
|
Untreated
Control
|
1.86
|
0.23
|
|
|
Gamma
|
3.235
|
1.07
|
|
Elongation
(%)
|
Supercritical
CO2
|
2.528
|
0.33
|
|
p
= 0.5306
|
Untreated
Control
|
1.884
|
0.26
|
|
|
Gamma
|
4.208
|
0.496
|
|
Strain
(%)
|
Supercritical
CO2
|
3.506
|
0.3724
|
|
p
= 0.5216
|
Untreated
Control
|
4.692
|
0.3445
|
|
|
Gamma
|
672.8
|
81.47
|
|
Load
(N)
|
Supercritical
CO2
|
683.1
|
72.39
|
|
p
= 0.154
|
Untreated
Control
|
812.2
|
86.46
|
|
|
Gamma
|
34.91
|
4.175
|
|
Tensile
Stress (MPa)
|
Supercritical
CO2
|
39.44
|
2.897
|
|
p
= 0.154
|
Untreated
Control
|
46.37
|
5.508
|
|
|
Gamma
|
3.728
|
0.33
|
|
Modulus
(MPa)
|
Supercritical
CO2
|
3.919
|
0.39
|
|
p
= 0.5690
|
Untreated
Control
|
4.3
|
0.33
|
|
|
Gamma
|
0.3
|
0.09
|
|
Fatigue
(%)
|
Supercritical
CO2
|
0.53
|
0.05
|
|
p
= 0.6543
|
Untreated
Control
|
0.49
|
0.09
|
Table 1. Results of
ANOVA calculations, Mean, and standard error for untreated
control, Gamma irradiated and supercritical CO2 treated BTBs.
Newton (N); Mega Pascal (MPa); Percent (%).
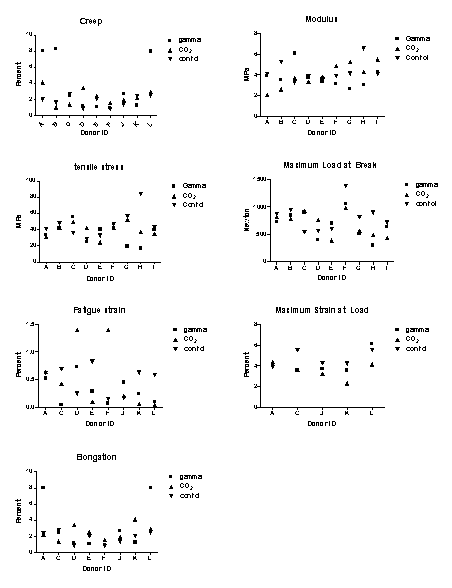
Figure
3. Graphs comparing effects of sterilization methods of
biomechanical properties of BTBs for all donors
Of the biomechanical parameters monitored in bone testing, no
statistically significant differences in density, ash fraction,
and Young’s modulus were observed in the three groups (Table
2). Three-point
bending revealed that gamma sterilization negatively impacted
the biomechanical properties with respect to flexural strength,
maximum load, and overall toughness (Table 2 and Figure 4).
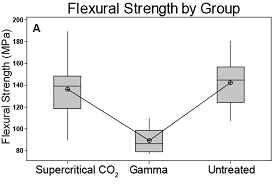
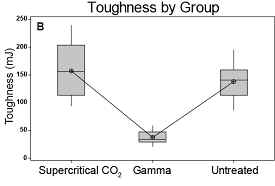
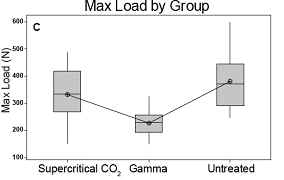
Figure
4. Box plots of those measures showing significant differences
between groups. A. Flexural strength, B. Toughness, and C. Max
load all are unchanged by supercritical CO2 sterilization but
compromised by gamma treatment.
|
|
Group
|
Number
|
Mean
|
StDev
|
|
Density
(g/cc)
|
Supercritical
CO2
|
12
|
1.94
|
0.05
|
|
Untreated
|
12
|
1.94
|
0.06
|
|
Gamma
|
11*
|
1.96
|
0.03
|
|
Ash
Fraction
(ash/dry)
|
Supercritical
CO2
|
12
|
0.646
|
0.011
|
|
Untreated
|
12
|
0.650
|
0.010
|
|
Gamma
|
12
|
0.649
|
0.010
|
|
Flexural
Strength
(MPa)
|
Supercritical
CO2
|
12
|
136.1
|
27.39
|
|
Untreated
|
12
|
142.05
|
20.09
|
|
Gamma
|
12
|
89.38
|
11.52
|
|
Toughness
(mJ)
|
Supercritical
CO2
|
9**
|
156.7
|
53.5
|
|
Untreated
|
9**
|
138.2
|
33.7
|
|
Gamma
|
9**
|
36.5
|
12.7
|
|
Max
Load
(N)
|
Supercritical
CO2
|
12
|
332.0
|
99.5
|
|
Untreated
|
12
|
379.9
|
100.0
|
|
Gamma
|
12
|
226.0
|
46.5
|
|
Young’s
Modulus
(MPa)
|
Supercritical
CO2
|
12
|
5320
|
1798
|
|
Untreated
|
12
|
5713
|
1231
|
|
Gamma
|
12
|
5866
|
1458
|
|
Hydroxylysyl
pyridinoline
(µmol/g)
|
Supercritical
CO2
|
12
|
8.7
|
3.1
|
|
Untreated
|
12
|
8.3
|
2.2
|
|
Gamma
|
12
|
9.3
|
2.8
|
|
Lysylpyridinoline
(µmol/g)
|
Supercritical
CO2
|
12
|
3.5
|
1.5
|
|
Untreated
|
12
|
3.3
|
1.1
|
|
Gamma
|
12
|
3.7
|
1.5
|
Table
2. Results of ANOVA calculations for Untreated, Gamma
irradiated, and supercritical CO2 treatment groups with respect
to the indicated measures. *one sample was removed due to
experimental error, ** Data from 3 independent samples per group
were excluded because clean breaks were not observed during
testing resulting in artificially inflated toughness.
Paired
T-testing confirmed no significant differences between control
(untreated) and SCCO2 sterilized cortical bone (Table 3).
By contrast, statistically significant differences were
noted between control and gamma irradiated bone (Table 2).
|
|
Flexural
Strength
(MPa)
|
Toughness
(mJ)
|
Max
Load
(N)
|
|
Untreated
|
142.1
|
138.3
|
379.9
|
|
Supercritical
CO2
|
136.1
|
145.0
|
332.0
|
|
Difference
|
6.0
p = 0.505
|
-6.7
p
= 0.58
|
47.9
p
= 0.136
|
|
Untreated
|
142.1
|
138.3
|
379.9
|
|
Supercritical
CO2
|
89.4
|
36.9
|
226.0
|
|
Difference
|
52.7
p
= 0.00
|
101.4
p
= 0.00
|
153.9
p
= 0.00
|
|
Untreated
|
136.1
|
145.0
|
332.0
|
|
Supercritical
CO2
|
89.4
|
36.9
|
226.0
|
|
Difference
|
46.7
p
= 0.00
|
108.1
p
= 0.00
|
106.0
p
= 0.00
|
Table
3 . Results of paired T-testing
for those measures with significant differences between groups.
Collagen
Cross-Linking
Analysis
of collagen cross-linking through measurement of
hydroxylysylpyridinoline and lysylpyridinoline revealed no
significant differences between control and test groups (Table
2). In
addition, no significant correlations were detected between any
of the biomechanical measures and the level of collagen
cross-links.
BTBs
Photographs
of sterilized BTBs (Figure 5) indicated that SCCO2
treatment produced significantly brighter and cleaner tendon
samples than either untreated or gamma irradiated samples.
Pre and post-sterilization weighing revealed that BTB
allografts lost approximately 14% of their mass.
That loss was attributed to extraction of lipids and
excess fluids during the sterilization process.
In fact, extracted lipid could be detected in the oily
residue present in the package after sterilization.
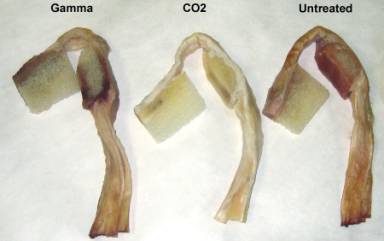
Figure
5. Photographs of bisected BTBs from a single donor with
respective sterilization treatments.
Discussion :
Modest, but
significant, differences were noted in the SCCO2 sterilization
of Allowashed and saline-rinsed tendon. In each case, samples
were inoculated with 106 CFUs of B. atrophaeus spores, adding to
whatever natural (presumably smaller) bioburden was already
present in the tissue samples prior to washing. A likely
explanation of these modest differences is that Allowashed
samples had an already diminished bioburden prior to
inoculation, as compared to saline-washed samples. However, the
more important conclusion is that no preprocessing is required
to achieve sterilization. Regardless of their preprocessing
history, tendon samples exposed to SCCO2 in the presence of
NovakillTM afforded sterile tissue, free of cellular or sporular
bacterial contamination.
Results from this study
also established that SCCO2 sterilization preserved the
biomechanical properties of the cortical bone. No significant
difference in all measures between SCCO2 sterilized and
untreated (Table 2, Table 3 and Figure 4) were apparent.
Preservation of these properties is important due to the fact
that grafts are largely used in load bearing orthopedic
applications. SCCO2 sterilization is effective at achieving
medical device levels of sterilization while maintaining
essential biomechanical properties.
Interestingly, while
some loss of lipid content was noted during tendon
sterilization, lipid extraction from the graft has been shown in
other studies with bone to increase graft incorporation
following transplant (14). Delipidation enhances accessibility
to microporous structures in the bone and enhances
osteoconduction following transplant (15), (16), (17). These
data demonstrated that besides achieving sterilization (SAL6),
SCCO2 treatment also preserved the biomechanical properties of
tendon.
Results reported here
show that effective (SAL6) terminal sterilization of bone and
tendon allograft tissue can be achieved while preserving
essential allograft properties and maintaining essential
biomechanical properties. Moreover, SCCO2 sterilized bone is
254% tougher, can withstand 68% greater load, and has 66% more
flexural strength than that of gamma irradiated samples.
SCCO2 sterilized
cortical bone is similar to untreated controls with respect to
pre-yield (elastic) and post yield (plastic) properties. This
observation is consistent with the preservation both of mineral
content, which is important for elastic properties, and
collagen, which is thought to preserve bone plasticity. No
significant differences in collagen cross-linking levels
relative to controls were noted in any of the test groups.
Moreover, the observation that gamma irradiated samples maintain
inherent Young’s modulus strength (an elastic property), but
exhibit reduced plasticity (i.e. toughness, flexural strength)
confirms earlier reports in the literature (5), (8).
A remaining concern
with respect to SCCO2 sterilization of bone allografts is the
osteoinduction and osteoconduction properties after
sterilization. The effect of sterilization on these processes
are not within the scope of the current study but will be
investigated in future studies. However, protein profiles and
content of Salmonella typhimurium inactivated using SCCO2
technology were unaffected when compared to protein profiles of
non-sterilized (control) cells (11). This coupled with our
current observations strongly suggests the gentle nature of this
process will preserve the essential properties of bone
allografts.
Tendon transplants
restore movement and flexion to patients suffering from loss of
function. This study demonstrates that SCCO2 sterilization can
be effective in achieving SAL6 (medical device level)
sterilization while maintaining the quality of the tendon
tissue. A remaining concern with respect to supercritical CO2
sterilization is the vascularization and cellular properties of
the tendon following transplant. Examination of cellular
structure by scanning electron microscopy (SEM) and transmission
electron microscopy (TEM) revealed no significant change in
cellular structure. Studies that definitively demonstrate
competence of tendon allografts in vivo are currently in
progress.
Adoption of SCCO2
sterilization by tissue banks will likely offer several
advantages, including the ability to perform terminal
sterilization in-house, preserve biomechanical properties of the
allograft, and reduce the need for microbiological
quantification of incoming bioburden. SCCO2 also makes possible
the use of industry standard validation methodologies, as with
medical devices, and possible eventual parametric release of
treated tissues. These advantages will facilitate continued
innovation and safe, high quality allografts by clearing
existing safety and biomechanical concerns associated with
current practices of tissue processing. Ultimately, the ability
to choose from a variety of sterilization options will increase
patient safety as well as positive surgical outcomes.
Reference :
-
United
States Bone and Joint Decade. Fast Facts on the Bone and
Joint Decade.
2006.
-
DePaula
CA, Truncale KG, Gertzman AA, Sunwoo MH, Dunn MG. Effects of
hydrogen peroxide cleaning procedures on bone graft
osteoinductivity and mechanical properties. Cell Tissue Bank
2005;6(4):287-98.
-
Arizono
T, Iwamoto Y, Okuyama K, Sugioka Y. Ethylene oxide
sterilization of bone grafts. Residual gas concentration and
fibroblast toxicity. Acta Orthop Scand 1994 Dec;65(6):640-2.
-
Thoren
K, Aspenberg P, Thorngren KG. Lipid extracted bank bone.
Bone conductive and mechanical properties. Clin Orthop Relat
Res 1995 Feb;(311):232-46.
-
Currey
JD, Brear K, Zioupos P, Reilly GC. Effect of formaldehyde
fixation on some mechanical properties of bovine bone.
Biomaterials 1995 Nov;16(16):1267-71.
-
Organ
Transplant and Grafts 1990 to 2009. Washington, DC: Census
Bureau; 2009. Report No.: 171.
-
Wang
S, Zinderman C, Wise R, Braun M. Infections and human tissue
transplants: review of FDA MedWatch reports 2001-2004. Cell
Tissue Bank 2007;8(3):211-9.
-
Akkus
O, Rimnac CM. Fracture resistance of gamma radiation
sterilized cortical bone allografts. J Orthop Res 2001
Sep;19(5):927-34.
-
Lomas
RJ, Gillan HL, Matthews JB, Ingham E, Kearney JN. An
evaluation of the capacity of differently prepared
demineralised bone matrices (DBM) and toxic residuals of
ethylene oxide (EtOx) to provoke an inflammatory response in
vitro. Biomaterials 2001 May;22(9):913-21.
-
Cornu
O, Banse X, Docquier PL, Luyckx S, Delloye C. Effect of
freeze-drying and gamma irradiation on the mechanical
properties of human cancellous bone. J Orthop Res 2000
May;18(3):426-31.
-
White
A, Burns D, Christensen TW. Effective terminal sterilization
using supercritical carbon dioxide. J Biotechnol 2006 Feb
20.
-
Fages
J, Poirier B, Barbier Y, Frayssinet P, Joffret ML, Majewski
W, et al. Viral inactivation of human bone tissue using
supercritical fluid extraction. ASAIO J 1998
Jul;44(4):289-93.
-
Fages
J, Poddevin N, King MW, Marois Y, Bronner J, Jakubiec B, et
al. Use of supercritical fluid extraction as a method of
cleaning anterior cruciate ligament prostheses: in vitro and
in vivo validation. ASAIO J 1998 Jul;44(4):278-88.
-
Lomas
R, Drummond O, Kearney JN. Processing of whole femoral head
allografts: a method for improving clinical efficacy and
safety. Cell Tissue Bank 2000;1(3):193-200.
-
Fages
J, Marty A, Delga C, Condoret JS, Combes D, Frayssinet P.
Use of supercritical CO2 for bone delipidation. Biomaterials
1994 Jul;15(9):650-6.
-
Kakiuchi
M, Ono K. Preparation of bank bone using defatting,
freeze-drying and sterilisation with ethylene oxide gas.
Part 2. Clinical evaluation of its efficacy and safety. Int
Orthop 1996;20(3):147-52.
-
Frayssinet
P, Rouquet N, Mathon D, Autefage A, Fages J. Histological
integration of allogeneic cancellous bone tissue treated by
supercritical CO2 implanted in sheep bones. Biomaterials
1998 Dec;19(24):2247-53.
|









