|
Abstract:
Anodized
implant surfaces which exhibit surface parameters like
roughness, micro porosities etc are found to result in faster
bone formation around a metallic implant. Cell adhesion on to an
implant surface is found to be based on protein adhesion
molecules and these molecules are learnt to favor a more
hydrophobic surface. In this study Ti6Al4V implant material was
anodized to varying levels and cell adhesion to these surfaces
were studied by a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl
tetrazolium bromide (MTT) assay. The degree of influence of
surface wet ability on cell adhesion was compared with other
surface parameters like roughness, micro porosities etc. The
anodized Ti6Al4V sample which showed maximum hydrophobic nature
also exhibited higher surface roughness and porosities which are
favorable for osteoblast adhesion and showed highest cell
viability. However it was noted that the anodized samples with
lesser hydrophobic nature than even the control surface of plain
polished Ti6Al4V also exhibited good cell adhesion
characteristics in the cell viability studies. It was concluded
that even though protein adhesion molecules favor a more
hydrophobic anodized surface ,the other factors like surface
roughness and micro porosities of surfaces compensates for a
lesser hydrophobic anodized surface to give appreciable cell
adhesion.
J.Orthopaedics 2009;6(1)e5
Keywords:
Ti6Al4V; Anodization; MTT assay; cell
viability
Introduction:
Titanium
and its alloys are the materials of choice for most dental and
orthopedic implants due to its biocompatibility and excellent
mechanical properties [1-3]. Among the Titanium alloys TiAl6V4
(Ti) is the most commonly used implant material [1-3].
Bone response and tissue integration with the implant material
depends on the physical and chemical properties of the surface.
Different surface modification techniques have been developed
for increasing the surface properties of (Ti), anodization being one of them[4].It has been well
established that the adhesion of osteoblasts on to an implant
surface is by the interaction of cell adhesion protein molecules
and these molecules favor surfaces with comparatively lesser
wetability, higher roughness
and porosities [5-8]. Anodized surfaces exhibit these
characteristics but these parameters vary with varying degree of
anodization. In this study Ti samples have been acid anodized at
different voltages to varying degree of anodization and the
influence of their surface properties and contact angle
measurements were studied to assess their protein adhesion
characteristics as compared to a control surface as well as an
acid etched surface (deoxidized). The results have been
counterchecked using a cell viability study of osteoblast cells
on the surface by an MTT assay and confocal imaging.
Materials
and Methods:
Medical
grade Ti disks of 15 mm diameter and 2mm thickness were cleaned
ultrasonically in acetone for 20 minutes and later cleaned in
70% ethanol solution and washed with distilled water. The
samples were etched in knolls reagent (2ml HF (40%) and 4 ml HNO3
(66%) in 1000 ml of water) and rinsed in distilled water and
dried in air. One set of etched sample was used for comparative
studies.
2.1
Procedure for anodization [9-10]
Set
up for anodization of the samples is shown in fig1.Ti was
anodized in 200 g/L sulfuric acid, 5% trisodium phosphate, and
5% sodium bicarbonate (baking soda). The electrolyte was
contained in a chemical resistant tank with Fume extraction. A
D.C. electrical supply with voltage regulation from 2 to
100 volts and sheet lead cathodes were provided.
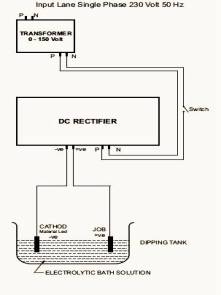 |
| Fig 1: Set up
for anodization of the Ti samples |
The
parts to be treated was immersed in the processing solution and
connected as the anode to the electrical D.C. source. The
temperature of the bath was maintained in the range of 20- 26°C
throughout the duration of treatment.
The
cell voltage was varied between 50-75 volts and three anodized
samples were obtained at 55 volts with yellow surface appearance
(sample labelled as yellow),60 volts with pink surface
appearance (sample labelled as pink) and 75 volts with blue
surface appearance (sample labelled as blue) respectively . The
time of treatment was 15 minutes for each sample. Immediately
after removal from the anodizing bath, parts were washed
thoroughly in clean running water, rinsed in clean hot water and
allowed to dry.
2.2 Characterization of Ti samples [11]
2.2.1
Film Thickness
The
thickness
of the anodized surfaces and average roughness was measured
using a Veeco Dektak 6M stylus profilometer.
2.2.2 Surface Morphology
Surface
morphology of the samples was examined using Scanning Electron
Microscope (SEM) (Hitachi S-2500, Tokyo, Japan).
2.2.3
Contact angle measurement
The
contact angles of the samples with three different fluids were
measured using a NRL contact angle goniometer (USA)
using the sessile drop method
[12] in three well characterized liquids, water, formamide and
di-iodomethane as per previous studies [13].
2.3
Cell Viability studies
2.3.1
Cell Culture
Osteosarcoma
cell line KHOS-NP (R-970-5) [NCCS] were grown in culture medium
(DMEM+10%FBS+1mM NEAA) in a T-25 flask and incubated at 37oC
for 2 days in a 5% C02
incubator (Thermo). ~ 5x105cells
were plated on to three samples each of anodized, etched and
plain polished control samples of Ti in 12 well plates. The
culture was incubated for 72 hours at 37oC
in 5% CO2
incubator. The samples with attached cells were used for MTT
assay and confocal microscopy.
2.3.2
MTT assay [14]
For
MTT assay, all the samples were transferred to a fresh plate and
800 mL
of MTT reagent was added to each well and incubated for 2 hours
at 37OC.
MTT transformed to dark blue formazan by mitochondrial
dehydrogenises enabling cell viability to be assessed. 800 mL
of lysis buffer (20%Sodium Dodecyl Sulphate 50% Dimethyl
Formamide 30% Distilled water) was added to each well, mixed and
incubated at 37oC
for 4 hours. 200 mL
of each sample was transferred to a fresh 96 well plate and the
optical density of the solution was measured at 570nm in an
ELISA microplate reader (Biorad USA). Analysis of optical
variance was used to evaluate difference in cell viability
between the groups [15].
2.3.3
Confocal Imaging
Osteosarcoma
cells were grown on another set of anodized etched and control
Ti samples in culture medium for 48 hours.
After which the cells were fixed with 4% Paraformaldehyde
( PFA), washed twice in 1X Phosphate Buffered Saline (pH 7.4)
(PBS) and incubated in DAPI (4’, 6-Diamidino-2-phenyindole)
(1:1000) for 10 minutes at room temperature (280C).
Cells were imaged for nuclear visualization thereafter using a
confocal microscope (Leica TCS SPE Germany).
Results
and Discussion:
The
plots of contact angle for the various implant surfaces are
shown in fig 2. As can be seen the contact angle for the
anodized sample (blue) at 75 volts is the highest in all fluids,
whereas the samples anodized at lower voltages (pink and yellow)
have lesser or comparable contact angles to that of the control
sample as
Fig
2: Plot of contact angles of the various samples in different
mediums.
as
well as the etched (deoxidized) sample. The plot of surface
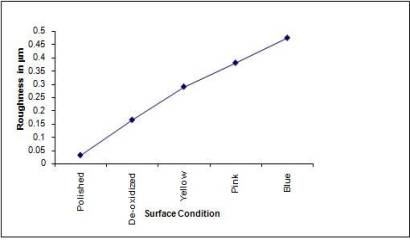
roughness and surface thickness of the samples are shown in fig
3 and fig 4. It is seen that the surface roughness and thickness
varies almost linearly with the degree of anodization.

Fig
3: Plot of average surface roughness of the various samples.
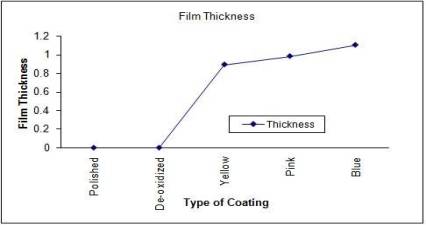
Fig
4: Plot of surface thickness of various samples
The
anodization thickness also increases linearly with the
anodization voltages. The scanning electron micrographs of the
various surfaces are shown in fig5-8.The sample anodized at the
highest voltage of 75 volts exhibit highest micro-porosities and
surface asperities followed by the ones anodized at lower
voltages. Etched sample (deoxidized) shows distinct etch line
topography.

5 |

6 |

7 |

8 |
|
Fig 5-8:
SEM micrographs of anodized and etched samples 5. Ti
anodized at 75 volts, 6.Ti anodized at 60 volts, 7.Ti
anodized at 55 volts, 8.HF etched Ti.
|
Variance analysis plot
for the different samples subjected to MTT assay is shown in fig
9.It is seen that the sample anodized at highest voltage of 75
volts (blue) exhibits maximum cell viability followed by the
sample anodized at lower voltages (pink and yellow) and the
etched (deoxidized) and control sample.
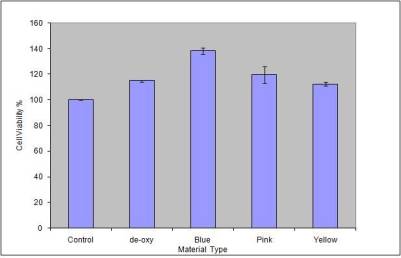
Fig
9: Optical variance analysis plot to assess the cell viability
of the samples
The confocal images of the
adhered nucleus to the various samples are shown in figs
10-14.
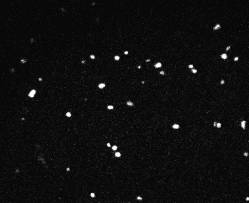
10 |
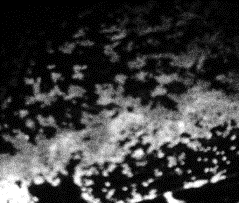
11 |
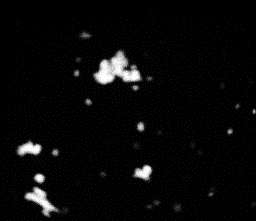
12 |
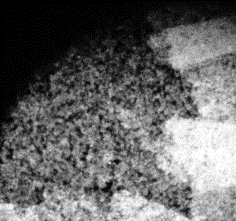
13 |
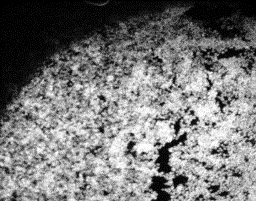
14 |
|
|
Fig 10-14:
Confocal Visualization of nuclear density on various
samples.Fig 10- Control TiAl6V4,Fig 11- Etched Sample,Fig
12- Anodized at 55V( Yellow),Fig 13-Anodized at 60
V(Pink),Fig 14-Anodized at 75 V(Blue). |
The
nuclear density as shown by the white patches is maximum for the
blue sample and decreases for the pink and yellow samples. The
etched sample showed nuclear density just lower than the pink
sample. The nucleus density confirms the maximum cell adhesion
characteristics of the sample anodized at 75 V followed by the
sample anodized at lower voltages and comparable nuclear density
for the etched sample to that of the pink sample. The cell
viability of the control sample is found to be the lowest.
As
made clear in previous studies, adhesion of cells on to
substrates occur through adhesion molecules which are proteins
[5-6] .These proteins favour adhesion to hydrophobic, rough and
porous surfaces. Since the Ti surface anodized at 75 V (blue)
exhibits highest hydrophobic nature, surface roughness and
maximum porosities, it presents the ideal condition for cell
adhesion and is established in the cell viability study. However
even though the wetability of the other anodized (pink and
yellow) and etched samples are only comparable or even less than
that of the plain polished control sample they exhibited higher
cell adhesion than the control sample. This can be attributed to
the higher roughness and porous natures of these samples in
relation with the control sample. Hence even though the adhesion
protein molecules which determine the adhesion characteristics
of cells on to an
implant surface is known to favour a hydrophobic surface ,the
other surface conditions like roughness, micro-porosities
,thickness are seen to override the dependence of cell adhesion
on hydrophobicity of an anodized implant surface.
Conclusions:
Ti
substrates were anodized to varying degrees and their surface
parameters for cell protein adhesion like wetability, roughness,
micro-porosity and thickness were studied and the influence of
wetability on cell adhesion was compared with the other surface
parameters thro a MTT assay and confocal imaging of adhered
nuclear density. An anodized surface which has the highest
hydrophobic nature also exhibited maximum roughness and porosity
and was seen to exhibit the highest cell adhesion
characteristics. However other anodized surfaces which exhibited
lesser hydrophobic nature than the control Ti surface also
exhibited good cell adhesion. This shows the dependence of cell
adhesion more on the surface parameters like roughness, micro
porosities and thickness within this range than on the
wetability of the surface. This factor can be considered for
selection of anodized implant material for orthopaedic use.
Further study is required to optimize for roughness and
hydrophobicity of anodized implant material.
Acknowledgements:
The
authors wish to place on record their gratitude to the various
research scholars of Indian Institute of Science, Bangalore,
India for having assisted in the contact angle measurements.
Special thanks to the research scholars of the Optoelectronics
laboratory of the Department of Physics Cochin University of
Science and Technology, Kerala, India and Rajiv Gandhi Centre
for Biotechnology Thiruvananthapuram, Kerala, India for
facilitating the various surface analyses. Timely advises and
suggestions given by Dr K.V.Menon, Amrita Institute of Medical
Science , Kerala, India and Dr H.K.Varma, Sree Chitra Thirunal
Institute of Medical Sciences Kerala, India are appreciated.
Reference :
-
C.F.Koch,
S.Johnson, D.Kumar, M.Jelinik, D.B.Chrisey, A.Doraiswamy, C.
Jin, R.J.Narayan, I.N.Mihailescu- Pulsed Laser Deposition of
Hydroxyapatite thin films. Materials Science &
Engineering C 27 (2007) 484-494.
-
Adriana
Bigi, Elis Boanini, Barbara Bracci, Alessandro
Facchini,Silvia Panzavolta,Francesco Segatti,Luigina Sturba-
Nanocrystalline hydroxyapatite coatings on titanium: a new
fast biomimetic method.. Biomaterials 26 (2005) 4085-4089.
-
C.K.Wang,
J.H.Chern Lin, C.P. Ju, H.C. Ong and R.P.H.Chang-Structural
characterization of pulsed laser deposited hydroxyapatite
film on titanium substrate. Biomaterials 18 (1997)
1331-1336.
-
Han-Jun
Oh, Jong-Ho Lee,Yonsoo Jeong,Young-Jig Kim,Choong-Soo Chi-
Microstructural characterization of biomedical titanium
oxide fabricated by electrochemical method. Surface and
Coatings Technology 198 (2005) 247-252.
-
G.Legeay
and F.Poncin-Epaillard Surface Engineering by coating of
Hydrophilic Layers: Bioadhesion and Biocontamination.
Adhesion –current research and application. , 2005 WILEY-VCH
GmbH & Co KGaA, Weinheim ISBN: 3-527-31263-3,175-188.
-
Kevin
Kendall –Molecular Adhesion and its Applications.2004
Kluwer Academic Publishers, New York, ISBN
0-306-46520-5,275-301.
-
S.R.Sousa,
M.A.Barbosa - Effect of Hydroxyapatite Thickness on Metal
Ion Release from Ti6Al4V Substrates .Biomaterials 17 (1996)
397-404
-
Despina
D. Deligianni , Nikoleta D.Katsala , Petros G. Koutsoukos ,
Yiannis F. Missirlis -Effect of Surface Roughness of
Hydroxyapatite on Human Bone Marrow Cell Adhesion,
Proliferation, Differentiation and Detachment. Biomaterials
22 (2001) 87-96.
-
J.Cl.Puippe-Surface
Treatments of Titanium implants. European Cells and
Materials Vol.5.Supl.1, (2003) 32-33. ISSN 1473-2262.
-
E.W.Russel
–Process specifications for Anodization of Titanium and
Titanium Alloys .Material Specification. Willsons Printers
(Leicester) Ltd London UK ISBN 0 11 470734 0.
-
P.S
Vanzillotta, G.A.Soares, I.N.Bastos, R.A.Simao, N.K.Kuromoto
- Potentialities of some surface characterization techniques
for the development of titanium biomedical alloys. Materials
Research, vol 7, no 3,437-444, 2004.
-
D
E Pacham- Handbook of Adhesion. John Wiley and Sons
Ltd, West susex, England. ISBN -13 978-0-471-80874-9, 79-85.
-
A.A.
Thorpe, Thomas G.Nevell, Simon A.Young, John Tsibouklis-Surface
Energy characteristics of poly (methylpropenoxyfluoroalkylsiloxane)
film structures. Applied surface science 136 (1998) 99-104.
-
Seunghan
Oh, Chiara Daraio, Li-Han Chen, Thomas R. Pisanic, Rita R.
Fin˜ ones, Sungho Jin - Significantly
accelerated osteoblast cell growth on aligned TiO2 nanotubes,
Wiley InterScience. DOI: 10.1002/jbm.a.30722
-
Jo-Young
Suh, Bong-Cheol Jang, Xiaolong Zhu, Joo L.Ong, Kyohan Kim-
Effect of hydrothermally treated anodic oxide films on
osteoblast attachment and proliferation. Biomaterials 24
(2003) 347-355.
|















