|
Abstract:
Objectives: To establish a protocol of OPCs culture
for cell transplantation to treat spinal cord injury and to
collect useful data about growth, differentiation, and
proliferation of OPCs, which are important for their therapeutic
effect of cell transplantation.
Methods: Mixed cells from cerebral cortices of
neonatal rats were cultured in vitro. Later, the OPCs were
separated by shaking process and differential adhesion. Then,
the OPCs were cultured in the conditional medium for
differentiation and proliferation. The growth pattern and
differentiation of OPCs were observed by microscopy and electron
microscopy. The maturation of OPCs was identified with
immunocytochemical technique and the proliferative ability of
OPCs was detected by MTT
(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)
assay.
Results: Around 9-10 days the distinct
stratification of glial cells well developed in primary culture.
Most of OPCs stayed and grew on the surface of astrocytes. The
OPCs were further separated by shaking process and differential
adhesion, and identified by the expression of specific marker.
Furthermore, it was proved that the OPCs were able to
differentiate into mature oligodendrocytes and proliferate in
vitro.
Conclusion: In this study, we have established the
rat OPCs culture in vitro. The OPCs stay at immature stage of
development and they are able to differentiate and proliferate
under certain condition in vitro, which are significant for the
therapeutic action after cell transplantation.
J.Orthopaedics 2008;5(4)e3
Keywords:
Oligodendroglia; cell culture; differentiation;
proliferation; spinal cord injury
Introduction:
Axonal demyelination is a common pathological change of a
number of diseases in central nervous system (CNS), including
brain trauma, multiple sclerosis, schizophrenia, normal aging,
and spinal cord injury (SCI) as well1,2. In SCI, both initial
insults and secondary injuries together cause the demyeliantion
of white matter3. Recently, it becomes clear that demyelination
of axons in SCI first takes place at the lesion epicenter, then
it chronically progresses in the adjacent white matter
fasciculus. Thus, it is proposed that intervention of axonal
demyelination have important therapeutic implication in the
treatment of SCI 4.
Myelin-forming cells in CNS exclusively come from
oligodendrocytes. It is likely that demyelination of axons have
close relationship with the dysfunction of oligodendrocytes in
SCI. Several experiments have presented convincing proofs
relative to the mechanisms which result in the death and
apoptosis of oligodendrocytes in wihte matter after SCI 5, which
further causes axonal demyelination and impairs the functional
recovery of injured spinal cord. Recently, some researches have
indicated transplantation of myelin-forming cells can facilitate
axonal remyelination and improve the neural function of injured
spinal cord 6,7. Oligodendrocytes play a vital role in both
facilitating the conduction of neural action potential and
supporting axonal survival. Oligodendrocyte precursor cells (OPCs),
the ancester of oligodendrocytes, can proliferate and migrate
throughout CNS during the late embryonic development, which can
differentiate into mature myelinating oligodendrocytes. As
immature cells, OPCs stay at the early stage of development of
oligodendroglial lineage cells, which have more potential to
differentiate and proliferate in vivo than that of mature
oligodendroctyes. Therefore, it is of great significance for the
functional recovery of SCI that OPCs are transplanted to improve
remyelination of survived axons and maximize the function of
injured spinal cord. In this study, we aim to establish an OPCs
culture in vitro to provide huge amount of myelinating cells for
cell transplantation. Furthermore, we also investigate the
important biological characteristics of OPCs in vitro to obtain
useful data for cell transplantation in the treatment of SCI.
Material and Methods :
Animal care
All experimental animals
were supplied by the experimental animal center of the Third
Military Medical University. All procedures were performed
according to institutional and governmental regulations, and in
accordance with the policy set and delineated by the animal care
committee of the University.
The culture of OPCs
Briefly, the 48-hour-old
Sprague-Dawley (SD) rats were anesthetized with an i.p.
injection of 1% sodium pentobarbital (50mg/kg), sprayed with 70%
ethanol and decapitated. Then, brains were removed under sterile
conditions with the aid of a dissecting microscope (Leica). The
basal ganglia, hippocampus, meninges and vessels were completely
removed. After washed in Hanks balanced salt solution (Gibco),
the meninges-free cerebral cortices were minced into 1 mm3 cubes
and dissociated into cell suspension. Passing through 74 μm cell
strainer, the filtrate was collected and centrifuged (1000 r/min
for 10 min, 4℃). The pelleted cells were re-suspended with basic
culture medium (BCM) [Dulbecco’s Modified Eagle Media(DMEM)(Gibco)supplemented
with 10% fetal bovine serum (FBS) (Gibco), 0.6% glucose (Gibco),
4 mmol/L L-glutamine (Amresco), 5 mmol/L sodium pyruvate (Amresco),
50 u/ml penicillinum (Gibco) and 50 ug/ml streptomycin (Gibco)]
and seeded to Poly-L-lysine (PLL) (Sigma)-coated flasks at the
concentration of (1.0–2.0)×106 cells/flask. Add BCM into flasks
and the flasks were transferred into a humidified incubator at
37℃ with 5% CO2. The cultures should not be disturbed in the
first 3 days, with BCM fed every 2–3 days after that.
The separation of OPCs was
usually carried out around 9–10 days in primary culture.
Secondary to additional 24-hour incubation with fresh BCM, the
flasks were placed onto a rotary shaker to remove microglia
(180rpm, 37ºC, 1–2h). Pour off the medium with dislodged cells,
wash flasks with phosphate-balanced saline (PBS) ( 0.01 M, pH
7.4) and add fresh BCM. In addition to 2-hour incubation, the
flasks were placed back onto the shaker again for overnight
shaking (200rpm, 37ºC, 18–20h). After the long-time shaking, the
supernatant was poured through 74 μm strainer and the filtrate
was collected and centrifuged (1000rpm, 4ºC, 10min). The
pelleted cells were re-suspended with BCM and seeded onto
uncoated culture dish for 1-hour incubation. Expel the medium
over the surface of dish several times to remove loosely
adherent cells, then collect the supernatant and centrifuge
again (1000rpm, 4ºC, 10min).
Then, the isolated OPCs
were re-suspended and further cultured in differentiation
culture medium [DMEM supplemented with 0.5% FBS, 50 ug/ml
transferrin (Sigma), 5 ug/ml insulin (Sigma), 30 nmol/L sodium
selenite (Sangon), 30 nmol/L thyroxine (Sigma), 4 mmol/L
L-glutamine, 5 mmol/L sodium pyruvate, 50 u/ml penicillinum and
50 ug/ml streptomycin] for differentiation or in OPCs culture
medium [Differentiation culture medium supplemented with 10 nmol/L
basic fibroblast growth factor (bFGF) (Peprotech) and 10 nmol/L
platelet-derived growth factor-AA (PDGF-AA) (Peprotech)] for
proliferation.
Observation of growth
pattern and differentiation of OPCs
Microscopy and scanning
electron microscopy
The growth pattern and
cellular morphology of OPCs in the primary culture and in the
differentiation culture were continuously investigated with
phase contrast microscope and scanning electron microscope
(Hitachi). For scanning electron microscopy (SEM) analysis,
primary cultures were terminated when cell stratification
distinctly formed in the culture. Cell cultures were rinsed with
PBS, fixed in 2.5% glutaraldehyde for 2 h and postfixed in 1%
osmium tetroxide for 1 h. Then, the samples were dehydrated in a
graded series of ethanol and further dried in t-butyl alcohol
for 5 min. After coated with gold-palladium, the specimens were
viewed with S-3400N Hitachi scanning electron microscope.
Immunocytochemistry
Here, we identified the
OPCs and their differentiation with immunocytochemistry. The
separated cells were seeded onto PLL-coated coverslips and
rinsed with PBS and fixed in 4% paraformaldehyde. After washed
with PBS, the coverslips were treated with 0.5% Triton X-100 for
10 min. Following blockage with 5% goat serum for 15 min, the
samples were incubated with rabbit anti-platelet-derived growth
factor receptor alpha antibody (anti-PDGPR-α) (1:100, Santa
Cruz) overnight at 4ºC. After washed in PBS, the coverslips were
incubated with FITC-conjugated goat anti-rabbit IgG (1:100,
Santa Cruz) for 1 h at 37ºC. The specimens were rinsed with PBS
and coverslipped with mounting medium.
The differentiation of OPCs
in vitro was also studied with immunocytochemical technique.
After the differentiation in the conditional medium, the OPCs
were immunostained with the specific antibody for mature
oligodendrocytes. The cell culture were labelled with the
primary antibody, myelin basic protein (MBP) (1:100, Santa
Cruz), overnight at 4ºC and visualized with FITC-conjugated goat
anti-rabbit IgG (1:100, Santa Cruz) for 1 h at 37ºC. The
negative controls used PBS instead of primary antibodies for
immunostaining.
The proliferation of
OPCs in vitro
The viability and
proliferation of OPCs in vitro were also detected by MTT assay
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide].
After the separation, the pelleted OPCs were re-suspended with
the OPCs culture medium and adjusted to the concentration of
5.0×104 cells/ml. Plate cells at 200 ul/well (~1.0×104 cells)
into 96-well tissue culture plate. After incubated for 24 h, a
total of 7 serial wells (each in triplicate) were further tested
for the following 7 consecutive days. The reagent of 20 ul MTT
(Sigma) (5mg/ml) was added to each well for color reaction, and
absorbance of the soluble formazan product in wells was measured
at wavelength of 492nm with 650nm as a reference, reading in a
plate reader (Tecan Model). Three control wells were added with
culture medium alone. The average values from the triplicate
wells were determined.
Statistical analysis
All data were expressed as
mean±standard deviation. The results of MTT assay were analyzed
using SPSS12.0.
Results :
The growth pattern and
morphology of OPCs in primary culture
Initially, the OPCs grew
scattered on the substratum of primary culture. Later on, some
OPCs migrated and grew onto the surface of astrocytes. The OPCs
were small in sizes, with round or oval shapes. Some of the OPCs
displayed fine cell processes. Around 9–10 days in primary
culture, the stratification of mixed glial cells distinctly
formed. The bed layer mainly consisted of flat and confluent
astrocytes (identified by immunolabelling, data not shown).
Meanwhile, most of OPCs grew clustered and scattered on the
surface of astrocytes (i.e. top layer), which represented the
typical growth pattern of OPCs in the primary culture. At this
time, most of OPCs displayed typical appearance of round or oval
shapes with two or three fine processes (Fig.1A).
Moreover, the SEM clearly
revealed the growth pattern of OPCs in the primary culture. The
OPCs were observed resting close on the surface of confluent
astrocytes. They had small and round soma, 6–10 μm in diameter.
Meanwhile, their cell bodies typically had two or three fine
cell processes. Otherwise, the confluent astrocytes of bed layer
had large, flat cell bodies and irregular shapes (Fig.1B). Such
a growth pattern of OPCs adhesive to astrocytes indicated there
was close relationship between the two types of cells.
After the separation, the
OPCs were further identified by immunocytochemistry. These
isolated OPCs significantly expressed the PDGPR-α which is the
lineage-specific marker of oligodendrocytes, and the control
showed the negative result (Fig.1C).
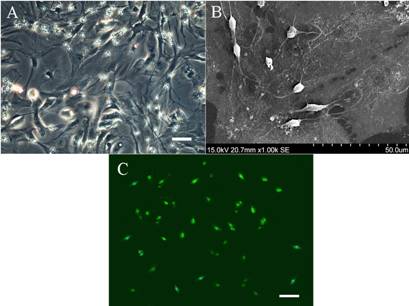
Fig 1: The
growth pattern and morphology of OPCs in culture.
(A)The stratification
of mixed glial cells in primary culture distinctly formed around
9–10 days in vitro. The bed layer were confluent
astrocytes and the OPCs grew clustered or scattered on the top
of the astroctye layer. Scale bar=50μm.
(B)The feature of
OPCs was further observed by scanning electron microscopy. The
OPCs were seen resting on the top of confluent and flat
astrocytes. Typically, the OPCs had small and round soma with
two or three fine cell processes. This growth pattern of OPCs
indicated the close relationship between the two different cell
types.
(C)The OPCs were
identified by immunocytochemistry. The shaken-off OPCs from
mixed glial cultures were further immunostained with the
specific cell marker of precursor cells, PDGPR-α. Scale
bar=50μm.
The
differentiation of OPCs in vitro
To study the differentiation
ability, the separated OPCs were further cultured in the
conditional medium for differentiation. Initially, the OPCs
displayed typical appearance of precursor cells, which only had
small round or oval cell bodies with few processes. Later on,
the OPCs progressively differentiated into mature
oligodendrocytes. After 1–2 days in the conditional culture, the
OPCs had extended delicate processes without apparent branching
(Fig.2A). After 3–5 days, this simple multipolar morphology of
OPCs had evolved to more complex forms, characterized by the
profuse outgrowth of elongated processes and extensive secondary
branching. At last, the mature oligodendrocytes displayed
typical appearance of “ramificated” or “cobweb-like” processes
reticulating in their periphery (Fig.2B). Correspondingly, the
expression of MBP, specific marker for mature oligodendrocytes,
further confirmed the differentiation of OPCs in vitro (Fig.2C),
and the result of control was negative. In all, these findings
demonstrated that OPCs retained the ability to differentiate
into mature oligodendrocytes in vitro.
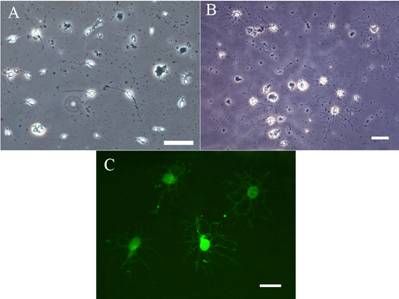
Fig 2: The
characteristics of OPCs differentiation in the conditional
medium
(A)Following the
shake-off process, the separated OPCs were further cultured for
differentiation. Initially, the OPCs typically displayed small
round or oval soma with few simple processes as the typical
appearance of precursor cells. Scale bar=50μm.
(B)After 3–5 days in
the culture, the morphology of OPCs developed into more complex
forms, characterized by the pattern of “ramificated” or
“cobweb-like” processes reticulating in their periphery. Scale
bar=50μm.
(C)The differentiated
oligodendrocytes were further identified by immunocytochemistry
as the expression of specific cell marker,MBP, correspondingly.
Scale bar=20μm.
The proliferation of OPCs in vitro
In this study MTT assay was performed to investigate the
proliferation of OPCs. MTT assay involves the use of
mitochondrial activity of live cells to convert MTT to formazan,
whose concentration can be measured spectrophotometrically. The
separated OPCs were plated in 96-well tissue culture plate for
proliferation. These OPCs continued to grow in the OPCs culture
medium. As a result, the mitochondrial activity of OPCs
increased gradually in the early stages, which indicated the
number of OPCs in wells rose progressively. Thereafter, the
absorbance of the wells peaked on the 6th day and slightly
decreased later (Fig 3). Here, the results of MTT assay revealed
the OPCs also retained the reproductive activity and were able
to proliferate in vitro.
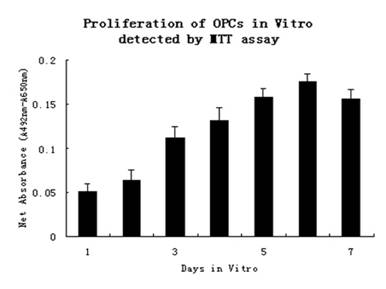
Fig 3:The
proliferation of OPCs in vitro detected by MTT assay.
The results of MTT
assay clearly revealed that the OPCs still maintained the
proliferative ability in vitro. Values represent specific
absorbance (A492-A650) of the formazan
product generated after incubation in MTT. Graph represents the
data (mean ± standard deviation) from triplicate wells.The
average absornance values were plotted on the y-axis with the
days of culture on the x-axis.
Discussion :
Myelin sheath supports fast nerve
conduction along axons8. Demyelination is one of the prominent
pathological changes in SCI, which further interferes with nerve
conduction. So, the normal function of myelin-forming cells and
myelin sheath is essential for the normal function of CNS.
Amelioration of axonal myelination is of great importance for
functional recovery of injured spinal cord 9,10. As the unique
myelinating cells in CNS, oligodendrocytes are reasonably
expected to be one of the suitable candidates for cell
transplantation to improve axonal myelination after SCI.
The culture of oligodendrocytes
is the prerequisite for the study of cell transplantation in
vivo. There are several distinct developmental stages of cell
differentiation identified for oligodendroglial lineage cells.
At different stages of development, oligodendroglial lineage
cells express stage-specific antigens, showing different
proliferative and migrative capacities and distinct
morphologies11. In this study we have developed a protocol of
OPCs culture with modification and improvement of previous
methods12. Since myelination of nervous system of rats peaks
around 3 weeks postnatally, the appropriate time to harvest OPCs
should be prior to complete maturation in order to ensure OPCs
are in active immature stages. Furthermore, the appropriate time
for the purification of OPCs should be also precise. In this
experiment, distinct stratification of mixed glial cells
developed around 9–10 days in primary culture, which represented
the very condition to separate OPCs. That the period of OPCs
growth in vitro is too short or too long is unfavorable for the
separation of OPCs later 12.
As differentiation and
proliferation of implanted cells are important for the
therapeutic effect of cell transplantation, the characteristics
of differentiation and proliferation of OPCs were also
investigated in vitro to provide useful data for transplantation
study in vivo. At present, differentiation of oligodendrocytes
is usually induced by the low-serum or serum-free chemical
conditional medium. The thyroxine in the conditional medium is
vital for the survival of oligodendrocytes13. Furthermore, the
trace element of selenium also plays an important role in the
differentiation of oligodendrocytes from immature stages to more
mature stages. It has been reported that the selenite is able to
up-regulate gene expression of proteolipid protein and
myelin-associated glycoprotein, which is important for
myelination14.
In this study, we induced
differentiation of OPCs in the low-serum conditional medium with
selenite and thyroxine, and investigated the development of OPCs
into mature oligodendrocytes in vitro. After cultured in the
differentiation medium, the morphology of OPCs underwent
characteristic changes from the simple multipolar appearance to
more complex appearance with profuse outgrowth of elongated
processes and extensive secondary branching. The morphological
changes correspondingly reflect the differentiation and
maturation of OPCs in vitro. Moreover, these mature
oligodendrocytes were also identified by immunostaining with the
specific marker of MBP, which indicated their ability of myelin
production. Altogether, these results demonstrated that the OPCs
still maintained the ability to differentiate into mature
oligodendrocytes in vitro.
Furthermore, another important
biological characteristic, the proliferative ability of OPCs,
was also studied in vitro. The results of MTT assay indicated
the OPCs retained the proliferative capacity in the conditional
medium. The PDGF-AA and bFGF in the medium are vital trophic
factors for the growth and proliferation of OPCs 15. In vivo,
the PDGF-AA and bFGF are usually generated by astrocytes and
neurons. As such, the survival and proliferation of OPCs in
vitro also need the existence of both factors. In this
experiment, the growth pattern of OPCs adhesive to the
astrocytes in primary culture suggested that the astrocytes were
likely to provide necessary growth substrate or cellular signals
for the survival and growth of OPCs. After the separation, the
PDGF-AA and bFGF were added in the conditional medium and the
proliferation of OPCs in vitro was accordingly observed. Thus,
in the future study of cell transplantation the survival and
proliferation of implanted OPCs can be enhanced by the provision
of PDGF-AA and bFGF.
Conclusion:
Overall, in the study we have
successfully established a protocol to culture the OPCs from
cerebral cortices of neonatal rats. Both appropriate primary
culture and timely shaking process are important for the
efficient separation of OPCs. These OPCs are also proved to
retain the abilities to differentiate into mature
oligodendrocytes and to survive and proliferate in vitro, which
are critical for the therapeutic effect of cell transplantation
to treat SCI in vivo.
Reference :
1.Kövari E, Gold G, Herrmann FR,
Canuto A, Hof PR, Michel JP, et al. Cortical microinfarcts and
demyelination significantly affect cognition in brain aging.
Stroke 2004; 35: 410-414.
2 Kakulas BA. The applied
neuropathology of human spinal cord injury. Spinal Cord 1999;
37: 79-88.
3 Hulsebosch CE. Recent advances
in pathophysiology and treatment of spinal cord injury. Advances
in Physiology Education 2002; 26: 238-255.
4 Totoiu MO, Keirstead HS. Spinal
cord injury is accompanied by chronic progressive demyelination.
Journal of Comparative Neurology 2005; 486: 373-383.
5 Casha S, Yu WR, Fehlings MG.
Oligodendroglial apoptosis occurs along degenerating axons and
is associated with FAS and p75 expression following spinal cord
injury in the rat. Neuroscience 2001; 103: 203-218.
6 Kocsis JD, Akiyama Y, Lankford
KL, Radtke C. Cell transplantation of peripheral-myelin-forming
cells to repair the injured spinal cord. Journal of
Rehabilitation Research & Development 2002; 39: 287-298.
7 Barnett SC, Riddell JS.
Olfactory ensheathing cell transplantation as a strategy for
spinal cord repair-what can it achieve? Nature Clinical Practice
Neurology 2007; 3: 152-161.
8 Morell P, Quarles RH, Norton
WT. Myelin formation, strucure and biochemistry. In: Siegel GJ,
Agranoff BW, editors. Basic Neurochemistry. New York: Raven
Press; 1995. p. 117–43.
9 McDonald JW, Belegu V.
Demyelination and remyelination after spinal cord injury.
Journal of Neurotrauma 2006; 23: 345-359.
10 Utzschneider DA, Archer DR,
Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells
enhances action potential conduction of amyelinated spinal cord
axons in the myelin-deficient rat. The Proceedings of the
National Academy of Sciences USA 1994; 91: 53-57.
11 Baumann N, Pham-Dinh D.
Biology of Oligodendrocyte and Myelin in the Mammalian Central
Nervous System. Physiological Reviews 2001; 81: 871-927.
12 McCarthy KD, de Vellis J.
Preparation of separate astroglial and oligodendroglial cell
cultures from rat cerebral tissue. Journal of Cell Biology 1980;
85: 890-902.
13 Jones SA, Jolson DM, Cuta KK,
Mariash CN, Anderson GW. Triiodothyronine is a survival factor
for developing oligodendrocytes. Molecular and Cellular
Endocrinology 2003; 199: 49-60.
14
Gu J,
Royland JE,
Wiggins RC,
Konat GW.
Selenium is required for normal upregulation of myelin
genes in differentiating oligodendrocytes. Journal of
Neuroscience Research 1997; 47: 626-635.
15 Bögler O, Wren D, Barnett SC,
Land H, Noble M. Cooperation between two growth factors promotes
extended self-renewal and inhibits differentiation of
oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. The
Proceedings of the National Academy of Sciences USA 1990; 87:
6368-6372.
|





