|
Abstract:
We report a patient presenting to an orthopaedic clinic with
bilateral Achilles tendon masses who was subsequently diagnosed
with cerebrotendinous xanthomatosis. This is a lipid-storage
disease secondary to a disruption in cholesterol metabolism. In
the absence of the key enzyme, sterol 27-hydroxylase, other
metabolites are increased such as cholestanol. This elevated
concentration results in characteristic clinical findings such
as bilateral cataracts, tendon xanthomas, and neurologic
impairments including debilitating cerebellar ataxia and
cerebral degeneration. Treatment with chenodeoxycholic acid (CDCA)
replenishes the key bile acid in humans and as a result prevents
the up-regulation of cholesterol and cholestanol synthesis. This
remedy may decrease the size of xanthomas; however, reversing
neurologic deficits is rarely successful. Ultimately, early
diagnosis and initiation of treatment is critical for the future
well being of these patients before permanent detrimental
effects take place.
J.Orthopaedics 2008;5(3)e13
Introduction:
Cerebrotendinous
xanthomatosis is a rare lipid-storage disease producing and
storing excessive cholestanol1,2. Various
presentations include cataracts, neurological dysfunction and
tendon xanthomas. The tendinous manifestations typically
precede the onset of neurological symptoms by decades1.
Due to a mutation of the CYP27 gene on chromosome 23,
the sterol 27-hydroxylase enzyme is rendered inactive and unable
to appropriately metabolize cholesterol to bile acids4,5.
Although not reversible,
the progression of neurologic demise associated with this
autosomal recessive disease can be arrested with appropriate
treatment6,7. Early diagnosis of this disease is
critical to the patientís successful long term outcome. Here we
report a patient with a mild presentation of cerebrotendinous
xanthomatosis presenting only with bilateral Achilles tendon
masses without neurologic deficit.
Case Report :
An 18-year-old female presented complaining of slowly
enlarging masses overlying both distal Achilles tendons (Fig.
1). They were soft and not tender to palpation, however she did
complain that closed countered shoes caused significant pain
over the area. Despite being treated with non-steroidal
anti-inflammatory as well as oral steroid regimens, growth
continued over the past six years.
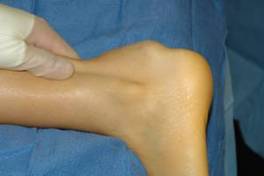
Fig. 1. Clinical preoperative
photograph of Achilles tendon mass.
Past medical history was
unremarkable for the most part. At age 6, bilateral cataracts
were treated with cataract removal and intraocular lens
placement. She has not suffered any developmental delay or
exhibited mental retardation. No other family members have
shown similar symptoms or signs of hypercholesterolemia.
Currently, she takes no medications and has no known drug
allergies.
On physical exam, each fusiform mass measured
8 cm in length starting approximately 2 cm proximal from the
Achilles tendon insertion. No neurologic impairment was noted
and she exhibited symmetrical 2+ deep tendon reflexes. Her
muscle strength, ankle range of motion and gait pattern were
unaffected. Close inspection did not disclose cutaneous
xanthomas or yellowish discoloration.
No osseous pathology was
noted on plain radiographs. Magnetic resonance images revealed
a soft tissue mass on each Achilles tendon exhibiting a
heterogenous signal (Fig. 2).
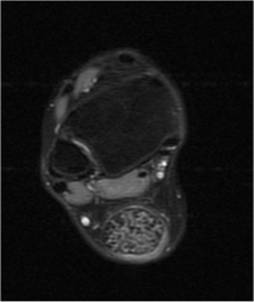
Fig. 2. Axial T2-weighted image
with fat suppression exhibiting a heterogenous signal within the
Achilles tendon.
Since non-operative
treatment failed, the patient requested surgical removal of both
swellings. The senior author (R.T.) agreed to remove one and
then assess her improvement and satisfaction before continuing
with the next. A posterolateral approach to the Achilles tendon
exposed a xanthoma (Fig. 3). Fatty yellow deposits infiltrated
the tendinous fibers; consequently, the mass could not be
completely excised. The Achilles tendon was debrided while
paying careful attention to minimize further insult to the
healthy fibers; essentially, the mass was debulked.
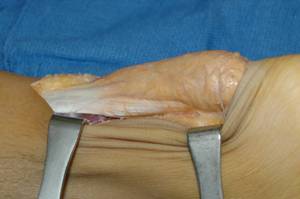
Fig. 3. Lateral
view of the exposed Achilles tendon xanthoma
The pathology department
prepared the tissue sample in formalin and examined it under
light microscopy. Innumerable foamy macrophages were visualized
with cleft-like spaces consistent with dissolved cholesterol
secondary to cellular processing. There were no indications of
malignancy (Fig. 4).

Fig. 4. A tissue
sample photomicrograph from the Achilles tendon exhibiting foamy
macrophages, cholesterol clefts, and multinucleatd giant cells
(Stain, hematoxylin and eosin, original magnification ◊200).
Liver enzymes and a lipid
panel found no abnormalities except for an elevated cholestanol
level of 28.9 ug/mL (normal value is 4.2 +/- 1.2 ug/mL). At
this point, a pediatric endocrinologist also evaluated her and
concurred with the diagnosis of cerebrotendinous xanthomatosis.
A treatment regimen was initiated immediately and consisted of
daily chenodeoxycholic acid as described by Berginer et al10.
Discussion :
Sterol 27-hydroxylase cleaves a cholesterol
side chain thereby yielding bile acids, specifically
chenodeoxycholic acid. In the absence of this enzyme, other
metabolites are produced i.e. cholestanol and bile alcohols. It
is theorized that without the negative feedback loop of bile
acids, cholesterol and cholestanol production are upregulated2.
This increased
concentration results in the characteristic clinical findings:
bilateral cataracts, tendon xanthomas, premature cardiovascular
disease and neurologic impairments.
Cataracts in the pediatric patient is usually the
earliest presentation of cerebrotendinous xanthomatosis.
Unfortunately, this can be mistaken for an isolated presentation
of congenital cataracts8. Although Achilles tendons
are the most common location, patellar and triceps tendon
xanthomas have been described as well7.
Neurologic presentation may
occur at any given time during the diseaseís natural history.
Key findings include cerebellar ataxia, dementia, pyramidal
signs, decreased intelligence, brain atrophy and seizure
disorder9. The exact mechanism in which cholestanol
produces neurologic dysfunction is unknown, however, this
disease process has been shown to up-regulate apoptotic pathways
thereby explaining the often noted cerebral atrophy10.
The earliest symptoms in an
infant consists of chronic diarrhea and cataracts followed by
tendon xanthomas in early adulthood11.
Immediately, the possibility of cerebrotendinous xanthomatosis
should be considered. In the presence of a positive diagnosis,
all family members should be screened as well. Molecular
genetic testing allows identification of heterozygotes and
therefore possibility for genetic counseling3.
When evaluating patients
with xanthomas and cataracts , the differential diagnosis should
include familial hypercholesterolemia12 and
sitosterolemia, a lipid disorder in which plant sterols are not
appropriately metabolized and therefore excessively deposited13.
Both disorders in effect are associated with elevated
cholesterol levels. Interestingly, neither result in neurologic
dysfunction further indicating that cholestanol itself directly
leads neural pathology.
In addition to the clinical
findings, diagnosis is confirmed primarily with laboratory
tests. Usually a lipid panel would be sufficient to reveal the
metabolic abnormality causing xanthomas, however, this patient
demonstrated normal plasma lipid and cholesterol levels. Only
the cholestanol was grossly elevated. Additional studies
showing elevated plasma and urine bile alcohol can also support
the diagnosis2.
Therapeutic options have
been developed based on cholesterol metabolism. Due to its lack
of production, bile acid replacement remains the cornerstone of
cerebrotendinous xanthomatosis treatment. Chenodeoxycholic acid
(CDCA) replenishes the key bile acid in humans and as a result
limits cholesterol, cholestanol and bile alcohol synthesis.
This remedy has been shown to halt neurologic deterioration as
well as decrease the size of Achilles tendon masses6,7.
Ursodeoxycholic acid, a bile acid commonly used for treatment of
primary biliary cirrhosis and small gallstones, is ineffective
for cerebrotendinous xanthomatosis. It is not actually a human
bile acid but instead developed commercially based on a bile
acid from the Chinese black bear. Because of this,
ursodeoxycholic acid is ineffective in providing the negative
feedback regulation14. There have been several
reports that 250mg CDCA three times daily results in successful
outcomes6,15; unfortunately, this has not always been
effective14.
Conclusion:
The case report we present
is unique in that cerebrotendinous xanthomatosis was diagnosed
in an adolescent presenting with bilateral Achilles tendons
despite having no neurological deficits. A goal of this paper
is to make orthopaedic surgeons aware of this disease process.
Ultimately, early diagnosis and initiation of treatment is
critical for the future well being of these patients.
Chenodeoxycholic acid may help decrease xanthoma sizes, however,
reversing neurologic deficits is rarely successful.
Reference :
1.
Bjorken I, Boberg KM, Leitersdoef E. Inborn errors in
bile acid biosynthesis and storage of sterols other than
cholesterol. In: Scriver Cr, Beaudet AL, editors. The metabolic
and molecular bases of inherited diseases. 8th ed.
New York: McGraw-Hill; 2001. p 2970-8.
2.
Moghadasian M. Cerebrotendinous xanthomatosis: clinical
course, genotypes and metabolic backgrounds. Clinical
Investigative Medicine 2004; 27: 42-50.
3.
Meiner V, Meiner Z, Reshef A, Bjorkhem I, Leitersdorf E.
Cerebrotendinous Xanthomatosis: molecular diagnosis enables
presymptomatic detection of a treatable disease. Neurology 1994;
44: 288-90.
4.
Cali JJ, Hsieh CL, Francke U, et al. Mutations
in the bile acid biosynthetic enzyme sterol 27-hydroxylase
underlie cerebrotendinous xanthomatosis. Journal of Biological
Chemistry. 1991; 266: 7779-83.
5.
Lee MH, Hazards S, Carpaten JD, Yi S, Cohen J. Gerhardt
GT, Salen G, Patel SB. Fine-mapping, mutation analyses, and
structural mapping of cerebrotendinous xanthomatosis in U.S.
pedigrees. Journal of Lipid Research 2001; 42: 159-169.
6.
Berginer VM, Salen G, Shefer S. Long term treatment of
cerebrotendinous xanthomatosis with chenodeoxycholic acid. New
England Journal of Medicine 1984; 311: 1649-52.
7.
Lamon-Fava S. Schaefer EJ. Garuti R. Salen G, Calandra S.
Two novel mutations in the sterol 27-hydroxylase gene causing
cerebrotendinous xanthomatosis. Clinical Genetics 2002;
61:185-91.
8.
Cruysberg JR, Wevers RA, van Engelen BG, Pinckers A, van
Spreeken A, Tolboom JJ. Ocular and systemic manifestations of
cerebrotendinous xanthomatosis. American Journal of Opthamology
1995; 20: 597-604.
9.
Federico A and Dotti MT. Cerebrotendinous Xanthomatosis:
Clinical Manifestations, Diagnostic Criteria, Pathogenesis, and
Therapy. Journal of Child Neurology. 2003; 18: 633-638.
10.
Inoue K, Kubota S, Seyama Y. Cholestanol induces apoptosis of
cerebellar neuronal cells. Biochemical and Biophysical Research
Communications 1999; 256: 198-203.
11.
Cruyaberg JR, Wevers RA, Tolboom JJ. Juvenile cataract
associated with chronic diarrhea in pediatric cerebrotendinous
xanthomatosis. American Journal of Opthalmology. 1991; 112:
606-607.
12.
Carranza-Bencano, A et al. Xanthomas of the Achilles Tendon:
Report of a Bilatreal Case and Review of the Literature. Foot
and Ankle International. 1999; 20(5): 314-316.
13.
Nguyen LB, Shefer S, Salen G. Molecular defect in cholesterol
synthesis in sitosterolemia with xanthomatosis. Journal of
Clinical Investigation 1990; 86: 926-31.
14.
Brodsky J, Beischer A, Dip Anat C, Soltero E, Tint S, Salen G,
Silverman J. Cerebrotendinous Xanthomatosis: A Rare Cause of
Bilateral Achilles Tendon Swelling and Ataxia. Journal of Bone
and Joint Surgery 2006; 88A: 1340-44.
15.
Batta AK, Shefer S, Batta M, Salen G. Effects of
chenodeoxycholic acid on biliary and urinary acids and bile
alcohols in cerebrotendinous xanthomatosis; monitoring by high
performance liquid chromatography. Journal Lipid Research 1985;
26: 690-8.
|






