|
Abstract:
We present the clinical review of a patient
of 20 years, diagnosed with Acute Lymphoblastic Leukaemia B, FAB
L2, treated by means of Pethema-LAL-RI protocol, who developed a
spondylodiscitis. We review the analytical, histological and
image studies made.
Objective: to demonstrate the existence of spondylodiscitis caused by Escherichia coli in neutropaenic
patients associated to acute lymphoblastic leukaemia.
Methods: We made clinical reviews over three
years, analysing the CAT, Rx, NMR, gammagraphy and
microbiological and histopathological studies.
Results: Spondylodiscitis caused by E.coli in
a neutropaenic patient with acute lymphoblastic leukaemia.
Antibiotic and antineoplastic treatments and orthopaedic
treatment by means of a Boston brace were administered with
favourable results.
Conclusions: In patients with neutropaenia
caused by anti-leukaemic chemotherapy, vertebral involvement is
exceptional, especially by Escherichia coli (6.6% of non-tuberculous
bacterial spondylodiscitis), since the few cases described are
of fungal origin, emphasising Candida, Scedosporium,
Blastoschizomyces or Aspergillus. We consider the great
importance of the current states of immunosuppression and their
vertebral repercussion. An early diagnosis, correct antibiotic,
antineoplastic and orthopaedic treatments allows the control of
this disease, reserving surgical treatment for the worst cases.
J.Orthopaedics 2008;5(3)e1
Keywords:spondylodiscitis,
Escherichia coli, acute lymphoblastic leukaemia, Boston brace.
Introduction:
Due to the increase of immunosuppressive
states unusual pathological processes arise. In patients with
neutropaenia caused by anti-leukaemic chemotherapy, vertebral
involvement is exceptional, especially by Escherichia coli,
since the few cases described are of fungal origin.
Case Report :
A 20 year old man was admitted in May 2002
for asthenia, haematomas and micro-adenopathies of 40 days
evolution. Haemogram: 11,100 leukocytes/ mm3, 70% of
blast cells of lymphoid nature. Bone marrow aspiration: massive
infiltration of lymphoid blasts, L2 morphology, hyperploidy of
50 chromosomes in mosaic (complex karyotype), t (9.22) negative,
bcr/abl negative: Acute Lymphoblastic Leukaemia Pre-B (F.A.B. of
L2). Corticoid therapy by means of Hickman type tunnelled
central catheter for inducer treatment according to
Pethema-LAL-RI protocol.
After 6 months of remission he was readmitted
for a fever of 40ºC and lumbalgia. A haemogram was made with
3,300 leukocytes/mm3, hypocellular marrow aspirate,
thoracic radiology, abdominal echography, NMR of lumbar spine,
gammagraphy, thick film, Rose Bengal normal. Blood cultures:
Escherichia coli sensitive to imipenem, ineffective against
persistent fever, necessitating levofloxacin.
CAT: appearance of abscessed lesions in both
psoas. CAT guided fine needle aspirate (FNA): haemopurulent
material with Escherichia coli. Change of the treatment to
cefotaxime and amikacine, with favourable evolution until
ambulatory discharge with ceftriaxone.
Two months later in the CAT the abscesses
persist in the psoas of 1.2 cm in the right side and of 2.6 cm
in the left side, with peripheral captation halo, marked
rarefaction in the vertebral bodies from L1 to S1 and
involvement of discs. (See Figure 1).
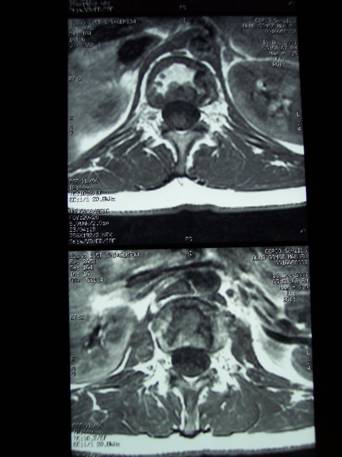
Figure 1. CAT:
abscesses in the psoas of 1.2 cm in the right side and 2.6 cm in
the left side, with peripheral captation halo, marked
rarefaction in the vertebral bodies from L1 to S1 and
involvement of the discs.
Results :
Spondylodiscitis caused by Escherichia Coli.
Two months later a new CAT was made: diminution of the space
between L1-L2 and L3-L4, hypodense areas, probable exostosis,
spondylolisthesis L3-L4 grade I and pattern of osteopenia. Rose
Bengal and Salmonella agglutination negative. Thoracolumbar
scoliotic posture, rectification of the thoracic kyphosis.
Lumbar kyphosis, good mobility, conserved osteotendinous
reflexes, bilaterally negative Lasègue and lumbar pain. Boston
brace is prescribed continuing with oral mercaptopurine,
methotrexate, ondansetron, ceftriaxone and levofloxacin.
A month later, negative Gammagraphy with
Technetium 99 and FNA, antibiotics being suspended.
A year later suffers viral oesophagitis
treated with foscarnet and Pneumocystis carinii pneumonia
treated with cotrimoxazole.
The patient is treated by Rehabilitation, a
riser wedge is prescribed and in March 2004, on obtaining normal
densitometry, ESR and CRP, the removal of the brace is decided.
Since then he has improved remarkably and at the moment he is
asymptomatic.
Discussion:
The increase
in patients with neutropaenia (neutrophils <500/mm3) due to
chemotherapy is more and more frequent (1), highlighting agents
involved in spondylodiscitis such as Candida, Scedosporium
apiospermum (2), Aspergillus (3-6)or Blastoschizomyces capitatus
(7;8). In fact, Park, considers that oncology patients,
neutropaenic through chemotherapy, with spondylodiscitis, as is
our clinical case, would be commonly affected by Candida and
Aspergillus (9). In fact Aspergillus even arises in
spondylodiscitis of bronchitics treated with corticoids (10).
With respect to the bacterial aetiology we found a series of
1780 cases of non-tuberculous Spondylodiscitis (NTS) between
1936 and 1992 (2).
S.aureus causes more than 50% of the NTS.
Streptococcus produces approximately 10% of the NTS.
E.coli follows causing 10-30% (11;12)of the NTS, or in 6.6% of
the cases (13). The portal of entry can be digestive,
urinary(14;15), biliary, cutaneous or pulmonary (hospitals).
Spread by blood. Spondylodiscitis has been described after
prostate biopsy (16), spinal surgery (17), diabetes or old
vertebral fractures (18). The Enterobacteria are emphasised in
the aged and Pseudomonas in iatrogenics and drug addicts. In a
study made in 1999 on 30 patients with spontaneous
spondylodiscitis focal endocarditis was found in 43.3%,
tuberculosis in 23.3%, urinary infection in 13.3%, focal
bacteraemia in 6.7% and without focus in 6.7%, The main
aetiologic organisms are considered to be Streptococcus in 33.3%
of the cases, Mycobacterium tuberculosis in 20%, Staphylococcus
spp. in 16.6%, Escherichia coli in 6.6% and Pseudomonas
aeruginosa in 6.6% with lumbar involvement in 60% of the cases,
dorsal in 26.6% and cervical in 13.3%, which are examples of the
main pathogeneses, aetiologies and locations (13).
Among the clinical manifestations are emphasised fever,
vertebral rigidity, radiculalgias, myositis of the psoas (19) or
exceptionally tetraplegia after manipulation of urinary tract
manipulations (20). In fact, in a study with 25 patients with
spondylitis, in spite of the neurological complications, the
results were favourable and the prognosis was positive (21;22).
Notable sequelae are recurrences, kyphosis and neurological
complications (23).
Regarding the diagnosis, it will be the clinical picture,
together with x-ray (24), CAT(25) or NMR, gammagraphy with
Technetium 99 or Gallium-67 citrate (26), FNA or biopsies.
Inflammation of the normal bone marrow, impingement of the disc
space, abnormality of paraspinal soft tissues and cortical
erosions(13) have been described in NMR. In fact, Ponte and
McDonald (15), described septic discitis in a woman of 77 years,
confirmed by NMR, and the FNA served to determine the agent and
its antibiotic treatment. The intradisc inoculation of bacterial
suspensions in dogs would cause vertebral fusions at 8 weeks,
with the most severe clinical picture being seen with
Staphylococcus and the least with Pseudomonas (27). It would
entail vascular proliferation, myxoid degeneration and necrosis
of the disc tissue causing a chronic osteomyelitis in the
proximities (28).
The empirical treatment would be the combinations of cloxacillin
or cefotaxime plus metronidazole or clindamycin; or beta-lactamics
plus aminoglycoside (gentamicin), or the combination of beta-lactamic
with fluorinated quinolones (ciprofloxacin) or the use of
aztreonam in monotherapy. Also the necessity of the drainage of
the abscesses in the psoas associated to antibiotic treatment
has been evaluated (18 ;29;30).
In 2003 the intradisc application of the combination of
gentamicin, cefazolin and clindamycin in the presence of iohexol
was considered for preventing discitis after diagnostic
procedures (31). Finally, corticoid therapy has been associated
to a greater risk of osteonecrosis (32) and chemotherapy to
growth, intellect, endocrine, cardiac and ocular alterations,
which complicates the clinical picture of these patients still
further (33).
Reference :
-
Digby JM,Kersley JB..Pyogenic non-tuberculous spinal
infection: an analysis of thirty cases. J Bone Joint Surg
Br.1979 Feb;61(1):47-55.
-
Ochiai N,Shimazaki C,,Uchida R et al. Disseminated
infection due to Scedosporium apiospermum in a patient with
acute myelogenous leukemia. Leuk Lymphoma.2003
Feb;44(2):369-72.
-
Takagi K,Yoshida A,Yamauchi T et
al. Successful treatment of
Aspergillus spondylodiscitis with high-dose itraconazole in a
patient with acute myelogenous leukemia. Leukemia.2001
Oct;15(10):1670-1.
-
Park KU,Lee HS,Kim CJ et al.. Fungal discitis due to
Aspergillus terreus in a patient with acute lymphoblastic
leukemia. J Korean Med Sci.2000 Dec;15(6):704-7.
-
Kawamura M,Takeuchi J,Hatta Y et
al. [Aspergillus lumbar discitis in a
patient with acute lymphoblastic leukemia following induction
therapy]. Rinsho Ketsueki.1995
Mar;36(3):206-11.
-
Cortet B,Richard R,Deprez X et al. Aspergillus spondylodiscitis:
successful conservative treatment in 9 cases.
J Rheumatol.1994 Jul;21(7):1287-91.
-
Ortiz AM,Sanz-Rodriguez C,Culebras
J et al. Multiple spondylodiscitis
caused by Blastoschizomyces capitatus in an allogeneic bone
marrow transplantation recipient. J
Rheumatol.1998 Nov;25(11):2276-8.
-
D'Antonio D,Piccolomini
R,Fioritoni G et al. Osteomyelitis and
intervertebral discitis caused by Blastoschizomyces capitatus in
a patient with acute leukemia. J Clin Microbiol.1994
Jan;32(1):224-7.
-
Stein DK,Sugar AM. Fungal infections in the
immunocompromised host. Diagn Microbiol Infect Dis.1989
Jul-Aug;12(4 Suppl):221S-228S.
-
Martinez M,Lee AS,Hellinger WC
et al. Vertebral Aspergillus osteomyelitis and acute diskitis in
patients with chronic obstructive pulmonary disease.
Mayo Clin Proc.1999 Jun;74(6):579-83.
-
Bontoux D,Codello L,Debiais F et
al. [Infectious spondylodiscitis.
Analysis of a series of 105 cases]. Rev Rhum Mal Osteoartic.1992
Jun;59(6):401-7.
-
David-Chausse J,Dehais J,Boyer M
et al. [Articular infections in
adults. Peripheral and vertebral involvement with common
bacteria and tubercle bacteria]. Rev Rhum Mal Osteoartic.1981
Jan;48(1):69-76.
-
Rivero MG,Salvatore AJ,de Wouters L. [Spontaneous
infectious spondylodiscitis in adults. Analysis of 30 cases].
Medicina (B Aires).1999;59(2):143-50.
-
Muller A. Bacterial spondylodiscitis after Escherichia
coli urinary tract infection. Dtsch Med Wochenschr.2001 Nov
16;126(46):1299-300.
-
Ponte CD,McDonald M. Septic discitis resulting from
Escherichia coli urosepsis. J Fam Pract.1992 Jun;34(6):767-71.
-
Koefoed-Nielsen J,Mommsen S. [Spondylodiscitis
as a complication to ultrasound-guided transrectal prostatic
biopsy]. Ugeskr Laeger.2002 Dec
30;165(1):51-2.
-
Muckley T,Schutz T,Kirschner M et
al. Psoas abscess: the spine as a
primary source of infection. Spine.2003 Mar 15;28(6):E106-13.
-
Rieker O,Duber C,Godderz W. [Spondylogenic psoas abscess:
long-term follow-up after percutaneous drainage]. Aktuelle
Radiol.1995 Mar;5(2):112-4.
-
Kerleau JM,Mejjad O,Lucet L et al.
[Abscess on the thigh, an unusual
complication of lumbar spondylodiscitis]. Presse Med.1992 Nov
14;21(38):1821.
-
Durance JP. Lumbar discitis in a patient with
quadriplegia: case report. Arch Phys Med Rehabil.1989
Mar;70(3):233-5.
-
Fica A,Bozan F,Aristegui M et al.
[Spondylodiscitis. Analysis of 25
cases]. Rev Med Chil.2003 May;131(5):473-82.
-
Thomachot B,Tonolli-Serabian I, Roux H. Spondylodiscites
infectieuses non tuberculeuses. -Encycl.Méd.Chir.(Elsevier,
París-France), Appareil locomoteur, 15-860-A-10, 1995,10p.
-
Beguiristain JL,Villas C,Garbayo A. [Non-tuberculous
infections of the spine]. Rev Med Univ Navarra.1987
Jul-Sep;31(3):149-52, 155-6, 159-61.
-
Laguna P,Moya M. [Abscess of the psoas muscle: analysis
of 11 cases and review of the literature]. Enferm Infecc
Microbiol Clin.1998 Jan;16(1):19-24.
-
Cordoba J,Pigrau C,Pahissa A et
al. [Psoas abscess: diagnostic and
therapeutic usefulness of echography and computerized
tomography]. Med Clin (Barc).1992 Nov
7;99(15):568-70.
-
Nolla-Sole JM,Mateo-Soria
L,Rozadilla-Sacanell A et al. Role of
technetium-99m diphosphonate and gallium-67 citrate bone
scanning in the early diagnosis of infectious spondylodiscitis.
A comparative study. Ann Rheum Dis.1992 May;51(5):665-7.
-
Ohno R. [Roentgenological and pathological studies on the
development of discitis in canine models]. Nippon Seikeigeka
Gakkai Zasshi.1991 Nov;65(11):1120-30.
-
Lucio E,Adesokan A,Hadjipavlou AG
et al. Pyogenic spondylodiskitis: a
radiologic/pathologic and culture correlation study. Arch Pathol
Lab Med.2000 May;124(5):712-6.
-
Penado S,Espina B,Francisco Campo J. [Abscess of the
psoas muscle. Description of a series of 23 cases]. Enferm
Infecc Microbiol Clin.2001 Jun-Jul;19(6):257-60.
-
Ampudia-Blasco FJ,Fernandez
J,Ferrer MD et al. [Psoas abscess
secondary to lumbar spondylodiscitis caused by gram negative
bacilli]. An Med Interna.1998 Aug;15(8):436-8.
-
Klessig HT,Showsh SA,Sekorski A. The use of intradiscal
antibiotics for discography: an in vitro study of gentamicin,
cefazolin, and clindamycin. Spine.2003 Aug 1;28(15):1735-8.
-
Mattano LA Jr,Sather HN,Trigg ME et al. Osteonecrosis as
a complication of treating acute lymphoblastic leukemia in
children: a report from the Children's Cancer Group. J Clin
Oncol.2000 Sep 15;18(18):3262-72.
-
Leung W,Hudson MM,Strickland DK et al. Late effects of
treatment in survivors of childhood acute myeloid leukemia. J
Clin Oncol.2000 Sep 15;18(18):3273-9.
|



