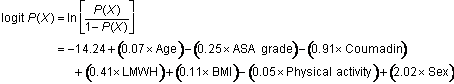| ORIGINAL
ARTICLE |
|
Risk Factors for Acute Pulmonary Embolism Following Total Hip and
Knee Arthroplasty |
|
Michael G
Walsh*,Charles Preston*, Vipul Patel*, Paul E DiCesare*.
*
Department of Orthopaedic Surgery, New York University Hospital for
Joint Diseases.
301 East 17th Street, New York, NY 10003
Address for Correspondence:
Michael Walsh
301 East 17th Street, Suite 1500
New York, NY 10003
Phone: 212-598-2459
Fax: 212-598-6096
Email: michael.walsh@nyumc.org
|
|
Abstract:
Pulmonary embolism (PE) is a potentially lethal complication
following joint replacement. Thirty out of 5,832 total hip or
knee patients at a single institution sustained an acute
post-operative pulmonary embolism. Multiple logistic regression
model showed that increasing age (odds ratio = 1.07, p = .004),
increasing body mass index (BMI) (odds ratio = 1.11, p < .001),
and female gender (odds ratio = 7.54, p = .05) were associated
with increased risk of PE. There were no statistically
significant differences in PE risk among DVT prophylaxis types
(p = .967). Based on the predictive models presented here using
our data, one can tentatively ascribe an overall level of risk
to patients prior to their total joint arthroplasty based on
patient BMI, age, and gender.
J.Orthopaedics 2008;5(2)e10
Introduction:
Pulmonary embolism (PE) is a potentially lethal complication
following joint replacement. Incidence rates of a venous
thromboembolic event (VTE) in the absence of prophylactic
measures of up to 63% have been reported following total hip
arthroplasty (THA) [1-6] and up to 40% following total knee
arthroplasty (TKA) [4, 7-9]. Although the widespread use of
pharmacological and mechanical prophylaxis since the 1970s has
led to a precipitous decline in these events [10-13], the
reported risk of fatal PE following arthroplasty nevertheless
remains 0.1–0.2% [10-12, 14, 15] regardless of which deep venous
thrombosis (DVT) prophylaxis regimen is undertaken [13, 16].
Risk factors for a VTE include increasing age [17], prior VTE
[18], smoking[19], lower extremity surgery [17], prolonged
immobilization[20], malignancy[21], hormone replacement
therapy[22], congestive heart failure [17], and the use of
general rather than regional anesthesia[23], although the
significance of these factors in conjunction with total joint
arthroplasty has not been studied. Selecting appropriate VTE
prophylaxis is thus complicated by the myriad of patients’
preoperative PE risk factor profiles.
The literature on PE prophylaxis after total joint arthroplasty
is of little help in this respect, due to the low incidence of
PE [24]. By comparison, the natural history and prevention of
DVT has been thoroughly investigated, since DVT incidence rates
are substantially higher than those of PE and thus cases of DVT
are more easily documented.
Reports showing a direct relationship between DVT and PE are
inconsistent[25, 26]. Absence of DVT has been noted in up to 30%
of patients diagnosed with PE[25]. The undiagnosed PE is of
clinical concern, and studies have shown that more than
two-thirds of all postarthroplasty deaths are due to PE[1].
Grady-Benson et al. have noted that the avoidance of pulmonary
embolic events should be the primary goal of VTE prevention[27].
Therefore, the focus of this investigation utilizes PE as the
study endpoint.
The purpose of this study was to determine the pre-, intra-, and
postoperative risk factors associated with an increased
frequency of clinically detectable PE following total hip or
knee arthroplasty. Identifying such risk factors may help us to
optimize VTE prophylaxis protocols and thus improve patient
outcomes after total hip or knee replacement.
Materials and Methods :
The study population consisted of 5832 patients undergoing
elective primary or revision total hip or total knee replacement
surgery at the authors’ institution from September 1997 to
February 2003. The Institutional Review Board approved this
study. Pre-, intra-, and postoperative variables from patient
charts (Table 1) were analyzed to identify possible risk factors
for the development of PE. Physical activity was rated using a
modified version of the University of California Los Angeles
(UCLA) Activity Level Scale [28], with 1 representing the lowest
level of activity and 5 the highest.
Table 1
Risk factor characteristics for pulmonary
embolism
|
Preoperative variables |
Intraoperative variables |
Postoperative variable |
|
Age |
Anesthesia type (general vs.
regional) |
DVT
prophylaxis type |
|
Gender |
Durations
of Surgery (OR Time) |
|
|
Body mass
index |
Estimated
blood loss |
|
|
ASA grade |
Tourniquet timea
|
|
|
Physical
activity level |
|
|
|
Presence of Diabetes |
|
|
|
Presence of Heart
Disease |
|
|
aTKA
patients only.
A total of 457 patients were tested for
suspected thromboembolic events during their acute inpatient
stay or acute rehabilitation stay following surgery. Patients
were considered to have a PE if they had a high-probability
reading on a ventilation/perfusion scan (the test most
frequently used for this purpose in our institution before 1999)
and/or a positive result on a CT angiogram (1998-2003).
Patients
were divided into groups corresponding to the method of DVT
prophylaxis administered them:
1.
Warfarin (coumadin) 5-10 mg started
the day before surgery and titrated to an INR of approximately
2.0 and continued for six weeks post-operatively;
2. Low-molecular-weight heparin (LMWH;
Lovenox, enoxaparin sodium, Rhone Poulenc Rorer, Collegeville,
PA) (subcutaneous injection of
either 40mg once per day or 30 mg twice a day) started
between 12 and 24 hr postoperatively and continued for 14
post-operative day;
3. Aspirin (325 mg each day) in
conjunction with foot pneumatic compression devices (Kendall AV
Impulse System, Mansfield, MA);
compression devises were discontinued upon discharge and aspirin
was continues for six weeks post-operatively.
Statistical Methods
We considered all potential risk factors
and confounders bivariately with PE status. For continuous
variables (age, American Society of Anesthesiologists [ASA]
grade, body mass index, physical activity, operating room [OR]
time, estimated blood loss [EBL], tourniquet time) the
differences between PE cases and controls were compared using
Student’s t-test, while for categorical variables
(gender, diabetes and cardiac disease status, joint, anesthesia
type, DVT prophylaxis), differences were compared using the
Fisher’s exact test (with significance defined as p ≤ .05
for both tests). Multiple logistic regression analysis was then
used to model the independent predictors of PE. Those variables
found to be significant in the bivariate analysis, as well as
any variables considered a priori as important confounders, were
included in this logistic model. The logistic model did not,
however, include the comorbidity variables (diabetes and cardiac
disease), as these were assessed differently for the cases and
controls.
Results:
The study population was 67% female, had a
mean age of 63 years, and comprised 2,665 THAs (1,679 unilateral
and 986 bilateral procedures; this group included 399 revision
procedures) and 3,167 TKAs (1,425 unilateral and 1,742 bilateral
procedures; this group included 190 revision procedures). Thirty
patients experienced a PE, 9 following THA and 21 following TKA;
the mean age of these patients was 71 years (range: 59-88
years); 24 were females and 6 males. The mean postoperative day
the PE was encountered was 4.2 (range 1-17).
Student t-tests performed on
continuous variables found that increasing patient age,
increased American Society of Anesthesiologists (ASA) grade, and
increased body mass index (BMI) were associated with an
increased risk of PE (Table 2). No association for either
increased or decreased risk for PE was found for patient
preoperative physical activity level, procedure length (OR
time), estimated blood loss (EBL), or length of tourniquet time
(TKA patients only).
Table 2
Two-sample t-test bivariate analysis
of characteristics with continuous variables.
|
Characteristic |
Controls |
Cases |
p |
|
Age,
years |
62.9
±
13.2 |
70.5
±
8.0 |
.0009* |
|
ASA grade |
3.37
±
0.86 |
3.06
±
0.81 |
.0372* |
|
Body mass
index, kg/m˛ |
30.4
±
7.2 |
36.0
±
6.4 |
<.0001* |
|
Physical
activity level |
2.55
±
0.68 |
2.41
±
0.56 |
.2370 |
|
OR time,
min |
139.8
±
369.4 |
123.8
±
37.4 |
.8185 |
|
EBL, cc |
402.0
±
491.7 |
276.7
±178.9 |
.1629 |
|
Tourniquet time,a
min |
77.2
±
47.2 |
72.1
±
32.5 |
.6023 |
aTKA
patients only; * p
≤ .05 for association with PE.
Fisher’s exact tests performed on
categorical variables revealed an increased risk of PE for both
female gender and TKA (Table 3). No association was found with
preoperative cardiac disease, preoperative diabetes, type of
anesthesia, or postoperative DVT prophylaxis.
Table 3
Fisher’s exact analysis of characteristics
with categorical variables
|
Characteristic |
No PE (n) |
PE (n) |
p |
|
Gender:
Male
Female |
3,860
1,895 |
7
23 |
.034* |
|
Diabetes |
311 |
4 |
.218 |
|
Systemic
cardiac disease |
1,280 |
12 |
.378 |
|
Arthroplasty type:
Knee
Hip |
3,146
2,656 |
21
9 |
.004* |
|
Anesthesia type:
General
Regional |
3,607
1,689 |
5
25 |
.454 |
|
DVT
prophylaxis:
Asprin/compression
boots
Coumadin
LMWH |
1,905
494
2,735 |
13
2
15 |
.967 |
*
p ≤ .05 for association with PE.
The multiple logistic regression model
showed that increasing age, increasing BMI, and female gender
were associated with increased risk of PE (Table 4).
Specifically, each year of advancing age was associated with a
7% increase in risk of PE (odds ratio = 1.07, p = .004).
Women experienced 7.5 times the risk for developing a PE as men
(odds ratio = 7.54, p = .05). An 11% increase in PE
incidence was associated with each kg/m2 increase in
BMI (odds ratio = 1.11, p < .001). There were no
statistically significant differences in PE risk among DVT
prophylaxis types (p = .967); post hoc power analysis
showed that 151 PEs would be required to demonstrate statistical
significance between prophylaxis types.
Table 4
Multivariate logistic regression analysis
|
Characteristic |
Odds ratio |
95% Confidence interval |
p |
|
Age |
1.07 |
1.02-1.12 |
.004* |
|
ASA grade |
0.77 |
0.43-1.40 |
.398 |
|
Female
gender |
7.54 |
1.003-56.70 |
.050* |
|
BMI |
1.11 |
1.07-1.17 |
<.001 |
|
Physical
activity level |
0.95 |
0.47-1.90 |
.883 |
|
DVT
prophylaxis:a
Coumadin
LMWH
|
0.40
1.50 |
0.05-3.24
0.63-3.60 |
.393
.357 |
aAspirin/compression
boots is the referent value; *
p ≤ .05 for association with PE.
The
following logistic model can be transformed to estimate the
probability of PE after specifying the covariate values for the
individual patient:
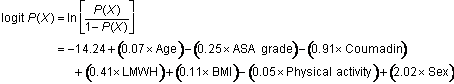
Once
transformed from the logit function, the following formula can
then be used to represent the probability of PE for a given set
of patient characteristics:
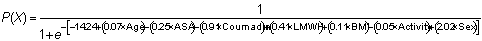
This equation allows the calculation of
individual probabilities of PE given specific patient profiles.
Discussion :
This study of over 5000 total joint arthroplasties utilizing
clinically detectable pulmonary embolism as an end point
evaluated several preoperative, intraoperative, and
postoperative potential risk factors for PE (Table 1).
Increasing patient age, female gender, and increasing BMI were
found to be independently associated with an increased risk for
pulmonary embolism. While many studies have compared various DVT
prophylaxes with the development of VTE, [10-13, 16] we found no
association between any of three methods of VTE prophylaxis and
greater or lesser risk of PE.
This retrospective observational study has both strengths and
weaknesses. We were able to analyze data related to more than
5000 total hip and knee arthroplasties by retrieving the data
gathered at the time of surgery and thereafter, which allowed
for precise statistical analysis. On the other hand, medical
comorbidities at the time of surgery were placed in categories,
but not recorded in detail. Thus all preoperative cardiac
conditions are classified as “systemic cardiac disease” and may
include congestive heart failure, arrhythmias, and hypertension.
Therefore, when assessing PE patients’ medical comorbidities,
direct comparison with the control cohort was not possible. Our
search of hospital records on ventilation perfusion scanning and
CT angiograms gave us an accurate assessment of patients who
experienced PE during the acute hospitalization or during their
stay in the acute rehabilitation unit; it did not, however,
capture any patient who had a PE after discharge to home or to a
different facility. Since the majority of PE events occur within
the first 14 days following arthroplasty [6, 29, 30], we are
reasonably assured that we have not substantially underestimated
the number of cases with clinically relevant PE. Furthermore, we
examined our population by discharge to home versus to
rehabilitative care to determine if there were substantive
differences in risk factor profile between these two
sub-populations (data not shown). While, those discharged to
rehabilitative care were older (mean age 65 vs. 60 yrs), they
were not significantly heavier, they were not in worse medical
condition (according to the American Society of
Anesthesiologists risk score), and they did not demonstrate any
difference in length of hospital stay. Moreover, we do not feel
that the difference in age between those discharged to home and
those discharged to rehabilitative care represents potential
bias in our results since all of our PE events were assessed
during the acute patient stay rather than a combination of
inpatient and rehabilitative patient accrual.
Our finding that increasing age is a risk factor for the
development of PE after total joint arthroplasty is supported by
White et al. [31], who noted that for each 10 years over the age
of 50, the rate of PE increased by 15% (odds ratio = 1.15; 95%
CI = 1.1-1.3). Lemos et al. showed that increasing age was
significantly associated with the development of pulmonary
embolism among patients undergoing a warfarin prophylactic
regimen following arthroplasty[32]. Mantilla et al., in a
retrospective study examining complications after total joint
arthroplasty, also noted an increase in PE among older patients
(p < .001) [33]. They found that embolic events were almost
twice as likely in patients in their eighth decade as in
patients in their sixth decade of life. This difference,
however, was only apparent between the extreme quantiles; they
did not find a linear increase in PE with increasing age.
Increased venous stasis, which may be most relevant for VTE, is
also a characteristic of increasing age [34].
It has not previously been shown that women have an increased
risk for pulmonary embolism following arthroplasty. The study by
Lemos et al. controlled for gender in their analysis of risk
factors for PE after total joint arthroplasty [32]. In a
separate study by Mantilla et al., the authors found no
difference in incidence of PE in male and female patients [33].
In our study group, 24 of the 30 PE events were in female
patients. BMI and age were both strong predictors of PE in our
study, and women had a significantly higher BMI (p < .001) and
were significantly older (p < .001) than men (data not shown).
While BMI and age contribute to some of the excess risk
experienced by women, gender differences nevertheless remained
even after controlling for these two risk factors (Table 4).
The finding that higher BMI is a risk factor for the development
of PE is supported by the work of Mantilla et al., who found a
50% increased risk of PE for each 5 kg/m˛ increase in BMI [30].
White et al. found that a BMI greater than 25 kg/m˛ was
associated with an increased risk in patients rehospitalized for
a VTE [35]. Obesity has been previously associated with
complications following total joint arthroplasty [36, 37]. It
has been postulated that obesity is associated with an increased
inflammatory state as well as a restriction in venous outflow
and blood propulsion [38]. These physiological alterations,
particularly in the presence of subclinical unstable
atherosclerotic lesions, may be the basis for the increase in
embolic events, and thus we believe that this represents a
strong patient risk factor for PE that should be considered by
the patient and surgeon.
Several conclusions that can be drawn from our study regarding
preoperative assessment must be further examined before they
deserve to be made recommendations. A multicenter approach may
allow investigators to acquire enough PE cases over a reasonable
time period. This type of study should allow for additional
stratification of various DVT prophylaxes and other risk factors
that predispose total arthroplasty patients to embolic events.
This is particularly important to determine the optimal intra-
and postoperative thromboprophylactic regimen in the presence of
specific risk factor profiles, something that our current lack
of statistical power would not permit.
Based on the predictive models presented here using our data,
one can tentatively ascribe an overall level of risk to patients
prior to their total joint arthroplasty based on the patient
BMI, age, ASA grade, and gender. We believe that these
statistically significant risk factors can be utilized in the
future to outline a more effective thromboprophylaxis following
total hip and total knee arthroplasty.
Reference :
- Haake, D.A. and S.A. Berkman, Venous thromboembolic disease
after hip surgery. Risk factors, prophylaxis, and diagnosis.
Clin Orthop Relat Res, 1989(242): p. 212-31.
- Stamatakis, J.D., et al., Femoral vein thrombosis
and total hip replacement. Br Med J, 1977. 2(6081):
p. 223-5.
- Turpie, A.G., et al., A randomized controlled
trial of a low-molecular-weight heparin (enoxaparin) to prevent
deep-vein thrombosis in patients undergoing elective hip
surgery. N Engl J Med, 1986. 315(15): p. 925-9.
- Geerts, W.H., et al., Prevention of venous
thromboembolism. Chest, 2001. 119(1 Suppl): p.
132S-175S.
- Lassen, M.R., et al., Prevention of
thromboembolism in 190 hip arthroplasties. Comparison of LMW
heparin and placebo. Acta Orthop Scand, 1991. 62(1):
p. 33-8.
- Johnson, R., J.R. Green, and J. Charnley,
Pulmonary embolism and its prophylaxis following the Charnley
total hip replacement. Clin Orthop Relat Res, 1977(127): p.
123-32.
- Lotke, P.A., et al., Indications for the treatment
of deep venous thrombosis following total knee replacement.
J Bone Joint Surg Am, 1984. 66(2): p. 202-8.
- Stulberg, B.N., et al., Deep-vein thrombosis
following total knee replacement. An analysis of six hundred and
thirty-eight arthroplasties. J Bone Joint Surg Am, 1984.
66(2): p. 194-201.
- Stringer, M.D., et al., Deep vein thrombosis after
elective knee surgery. An incidence study in 312 patients. J
Bone Joint Surg Br, 1989. 71(3): p. 492-7.
- Brookenthal, K.R., et al., A meta-analysis of
thromboembolic prophylaxis in total knee arthroplasty. J
Arthroplasty, 2001. 16(3): p. 293-300.
- Freedman, K.B., et al., A meta-analysis of
thromboembolic prophylaxis following elective total hip
arthroplasty. J Bone Joint Surg Am, 2000. 82-A(7): p.
929-38.
- Lieberman, J.R., et al., The efficacy of
prophylaxis with low-dose warfarin for prevention of pulmonary
embolism following total hip arthroplasty. J Bone Joint Surg
Am, 1997. 79(3): p. 319-25.
- Sarmiento, A. and A.D. Goswami, Thromboembolic
prophylaxis with use of aspirin, exercise, and graded elastic
stockings or intermittent compression devices in patients
managed with total hip arthroplasty. J Bone Joint Surg Am,
1999. 81(3): p. 339-46.
- Lieberman, J.R., et al., Low-dose warfarin
prophylaxis to prevent symptomatic pulmonary embolism after
total knee arthroplasty. J Arthroplasty, 1997. 12(2):
p. 180-4.
- Westrich, G.H., et al., Meta-analysis of
thromboembolic prophylaxis after total knee arthroplasty. J
Bone Joint Surg Br, 2000. 82(6): p. 795-800.
- Stern, S.H., R.L. Wixson, and D. O'Connor,
Evaluation of the safety and efficacy of enoxaparin and warfarin
for prevention of deep vein thrombosis after total knee
arthroplasty. J Arthroplasty, 2000. 15(2): p. 153-8.
- Anderson, F.A., Jr. and F.A. Spencer, Risk factors
for venous thromboembolism. Circulation, 2003. 107(23
Suppl 1): p. I9-16.
- White, R.H. and M.C. Henderson, Risk factors for
venous thromboembolism after total hip and knee replacement
surgery. Curr Opin Pulm Med, 2002. 8(5): p. 365-71.
- Goldhaber, S.Z., et al., A prospective study of
risk factors for pulmonary embolism in women. Jama, 1997.
277(8): p. 642-5.
- Kim, Y.H. and V.E. Kim, Factors leading to low
incidence of deep vein thrombosis after cementless and cemented
total knee arthroplasty. Clin Orthop Relat Res, 1991(273):
p. 119-24.
- Piccioli, A., et al., Cancer and venous
thromboembolism. Am Heart J, 1996. 132(4): p. 850-5.
- Daly, E., et al., Risk of venous thromboembolism in
users of hormone replacement therapy. Lancet, 1996. 348(9033):
p. 977-80.
- Sharrock, N.E., et al., Effects of epidural
anesthesia on the incidence of deep-vein thrombosis after total
knee arthroplasty. J Bone Joint Surg Am, 1991. 73(4):
p. 502-6.
- Bruce, W., et al., Occurrence of pulmonary
thromboembolism immediately after arthroplasty. Nucl Med
Commun, 2001. 22(11): p. 1237-42.
- Wolf, L.D., W.J. Hozack, and R.H. Rothman,
Pulmonary embolism in total joint arthroplasty. Clin Orthop
Relat Res, 1993(288): p. 219-33.
- Kim, Y.H., S.H. Oh, and J.S. Kim, Incidence and
natural history of deep-vein thrombosis after total hip
arthroplasty. A prospective and randomised clinical study. J
Bone Joint Surg Br, 2003. 85(5): p. 661-5.
- Grady-Benson, J.C., et al., Postoperative
surveillance for deep venous thrombosis with duplex
ultrasonography after total knee arthroplasty. J Bone Joint
Surg Am, 1994. 76(11): p. 1649-57.
- Amstutz, H.C., et al., Treatment of primary
osteoarthritis of the hip. A comparison of total joint and
surface replacement arthroplasty. J Bone Joint Surg Am,
1984. 66(2): p. 228-41.
- Westrich, G.H., et al., The incidence of venous
thromboembolism after total hip arthroplasty: a specific
hypotensive epidural anesthesia protocol. J Arthroplasty,
1999. 14(4): p. 456-63.
- Mantilla, C.B., et al., Risk factors for clinically
relevant pulmonary embolism and deep venous thrombosis in
patients undergoing primary hip or knee arthroplasty.
Anesthesiology, 2003. 99(3): p. 552-60; discussion 5A.
- White, R.H., et al., Incidence and time course of
thromboembolic outcomes following total hip or knee arthroplasty.
Arch Intern Med, 1998. 158(14): p. 1525-31.
- Lemos, M.J., et al., Pulmonary embolism in total
hip and knee arthroplasty. Risk factors in patients on warfarin
prophylaxis and analysis of the prothrombin time as an indicator
of warfarin's prophylactic effect. Clin Orthop Relat Res,
1992(282): p. 158-63.
- Mantilla, C.B., et al., Frequency of myocardial
infarction, pulmonary embolism, deep venous thrombosis, and
death following primary hip or knee arthroplasty.
Anesthesiology, 2002. 96(5): p. 1140-6.
- Lowe, G.D., Virchow's triad revisited: abnormal
flow. Pathophysiol Haemost Thromb, 2003. 33(5-6): p.
455-7.
- White, R.H., et al., Predictors of
rehospitalization for symptomatic venous thromboembolism after
total hip arthroplasty. N Engl J Med, 2000. 343(24):
p. 1758-64.
- Miric, A., et al., Perioperative morbidity
following total knee arthroplasty among obese patients. J
Knee Surg, 2002. 15(2): p. 77-83.
- Jain, N.B., et al., Comorbidities increase
complication rates in patients having arthroplasty. Clin
Orthop Relat Res, 2005(435): p. 232-8.
- Visser, M., et al., Elevated C-reactive protein
levels in overweight and obese adults. Jama, 1999. 282(22):
p. 2131-5.
|
|
This is a peer reviewed paper Please cite as
: Michael G Walsh: Risk Factors for Acute Pulmonary Embolism
Following Total Hip and Knee Arthroplasty
J.Orthopaedics 2008;5(2)e10
URL:
http://www.jortho.org/2008/5/2/e10 |
|
|