|
Abstract:
Objective: To establish stable enhanced green fluorescent
protein (EGFP)-expressing human osteosarcoma cell sublines with
different metastatic potential and investigate their biological characteristics.
Method: The pEGFP vector, which contains an enhanced GFP gene,
was transfected into human osteosarcoma MG63 cell line .Then
obtain two cell sublines of clone MG63-M6 and MG63-M8 with
different metastatic potential . Per cell lines proliferation
invitro,soft
agar formation,growth curve,nude
mice tumor formation test aggregatedly analyze its biological
behaviour.
Result: M6
and M8 two lines both kept anthro-chromatoid karyotype,the tumor formationed presents human osteogenic sarcoma epithelium
organizational shape, among which the population doubling time
of M6 is 38.4h,soft
agar formation rate is 18.7%,M8 population
doubling time is 23.0h,soft
agar formation rate is 29.3%. Hypodermically innoculate M6 and
M8 on the back of nude mice,detect the tumor
formation time of M8 is short,cell
proliferation is fast,but both don’t develop
transfection in 4w.
Conclusion: Osteosarcoma
cell subseries have different metastatic characteristics,the
integtation and expression of GFP have not posed any marked
influence on the growth state of MG63 cell,it can
be conducted and acted as the report gene to further comprehend
the variability analyzation of osteosarcoma cell metastasis.
J.Orthopaedics 2008;5(1)e6
Keywords:
Osteosarcoma; Neoplasm metastasis; Green fluorescent
protein
Introduction:
Green fluorescent
protein (GFP) gene is a kind of newstyle report gene [1].The
influence of human osteosarcoma MG63 cell which uses GFP
“mark” on the bionomics of MG63 cell and
differentiation invitro,law of development is not
reported yet domestically,so we progress comparative
study on the bionomics of the two cell lines through the
construction of vecter which contains enhanced green fluorescent
protein (EGFP) gene to transfect MG63,together obtain two
strains of MG63 monoclone sublines which possess different
metastasis potentiality
Material and Methods :
1 Material and instrument Human osteosarcoma: MG63 cell line (No.ATCC:CRL-1427)
bought from Cell Conservation Center of Wuhan University,enhancement
type GFP eukaryotic expression plasmid pEGFP-N1 is
favorably given by Doc. Rao Yaojian of our room.
RPMI -1640,fetal
calf serum,OPTI-MEM serum-free medium,liposome
transfection kit(Lipofectamin2000),HEPES
bought from Gibco Ltd.,
agar powder powdered(Sigma
subpackage),pancreatin,trypan
blue,DMSO,G418,PI
bought from Sigma Ltd.;CO2
gas incubator (USA);inverted
microscope (Olympus,Japan);EL
800 symase immunodetection instrument (Bio-Tek Ltd.,USA);PACE5000
electrophoretic apparatus(BeckMan,USA).
Nude mice BALB/c nu/nu,bought
from Laboratory Animal Center o f Tongji Medical College,4~6w’s
age,weight
18~
22g ,SPF
conditional breeding.
The extraction and identification of the
plasmid:the
preparation,purification of Bacillus coli
competent cells and the plasmid identification are progressed
according to routine method,the
plasmid extraction and purification is progressed according to
the instruction of the kit.
2 The cell culture and the plasmid
transfection: MG63 cell is
cultured by using RPMI1640 medium equally assisted with 10%
fetal calf serum,PG,streptomycin
100ug/ml each,in
the temp. of
37℃ ,5%CO2 . Undertake G418 minimal lethal concentration test
2w’s before transfection. Crop the MG63 cells which are in the
good condition of log phase,transfer
to 12-well board
according to the density of 1×105cells/bore,put
in
37℃
,5%CO2 incubator
cultured for 24h. Throw away cell culture liquid as soon as cell
adherence grows to 80% confluence,cleaning
cell for one time by serum-free medium, use the extractioned purified pEGFP-N1 plasmid to transfect MG63 cell
according to the instruction of liposome transfection kit,change culture fluid 4h’s after
transfection,
use G418 culture fluid which contains 600ug/ml to culture,after
48h observe the condition of transient expression,change
to G418 which contains 400ug/ml to maintain screening when most
part of the control
group’s cells are died,change
liquid to passage every 2~3d,resistance
clone is initially formed 2 w’s later,observe its intensity of green
fluorescence under Fluophot. When cell conjugation reaches to
80%,move
the cells to 6-well board to culture,until
passaging amplification revealed in culture flask,together collect cells to do every
item of experiments on the exponential phase of cell.
3 Monoplast
clone separation: Use limiting dilution assay,take
1×105/ml
MG63 cell through trypsinization ft cell suspension,the
density is 10pieces /ml,25pieces/ml innoculation 96- bore
culture board,
add 100ul cell suspension in each bore,average
0.5~2.0 pieces of cells /bore,together 5 culture boards,
add 100ul culture fluid in every bore. 4~5d after cloning choose
the bore which only contains single cell growth,add mark on the bore which is confirmed of containing single cell,observe monoplast colony formation
in the mark bore. Change culture fluid every 4~5d., Remove cells
to 24-well board to amplication culture as cell proliferation
area reaches to 1/3 of the wells,until
culture passage in the culture flask . Obtain 12 strains
monoclone stocks which are denominated M1~M12.
4 The determination of cell
electrophoresis module: Take M1~M12 quaque monocell clone strain to ft cell
suspension,density adjusted to 1.0×108/L( use trypan
blue rejection test to demonstrate ultimately the suspension
contains above 95% of living cells),admove into thawing
fusing type quartacapillary electrophoresis instrument,in
15kV voltage, 10s pressure introduction,detect the speed
of cell electrophoresis in the temp. of
37℃
,therefrom obtain the two strains which has the highest
electophoresis speed and the lowest electrophoresis speed as
well as maternal plant to progress the following experiments .
5 Chromosome analysis: Take 3 strains of above cells which have passaging grown
for 48h,add
Colchicine to the final concentration of 0.05ug/ml on
experimental day,harvest
after 6h’s effect,per hypotension,fixation,dropping
sheet,make
Giemsa and G strap coloration, under the
immersion objective each count 100 cells’ chromosome number,moreover
choose 20 pieces of scattered metaphase image to do chromosome
karyotype analysis(
calculate mode numerus,analyse karyotype after G banding
dyeing).
6 Cell
morphological check: Dropwise three strains of cells
onto glass slice,
culture for 48h in the plate which contains culture fluid,
HE dyeing ,light
microscope observation after kryo-acetone fixation; obtain
another two strains
of cells centrifugalized at the speed of 2000r/min,10min later,cell mass
fixed with 4.5% GA to make transmission electron microscope (TEM
) collection.
7 The observation of growth curve (MTT chromatometry) Inoculate
three strains of
cells by the quantity of 1×103/bore
into 96-well board, 24 bore per strain,culture
in incubator,add MTT(5mg/ml) dye 20ul every 24h,
in the temp. of
37℃
continue incubating 4h and then terminate culture,suction
the supernate of every hole,add 150ul DMSO,swing
for 10min,then
determine OD value of 490nm division,cum time(day)
to be abscissa axis,photo-absorbtion
value(A)as
YAS to draw growth curve.
8 The determination of clone formation:
Use soft agarose cloning technique,take three different density strains of cells which are incubated in
the temp. of
37℃
ft suspension, take 9.4ml
shift in tiny beaker, add
50℃
5% agar 0.6ml,quickly
misce bene,immediately spray into the two
24-bore culture boards which are paved by bottom-layer agar (density 0.3%),the cells of every strain add into
three bores each refer to the cell number of 100 and 50, add 0.8ml in
each bore,put
in room temperature until agar coagulation,
culture in incubation box for 14d in the temp. of
37℃
,5%CO2,observe
colony formation state under micro as well as counting the clone
number,the
cell number of each colony≥50 is one
clone.
9 The
experiment of nude mice tumor formation in vivo: Use RPMI1640
which contains no serum to prepare the cell suspension with the
density of 1×107pieces/ml after the digestion and ablution of the two lines and
stock plant,innoculate neoplastic cell on the
back of nude mice subcutaneously, 0.3ml each, 6 mice in
each,continually
observe for 6 w from the injection day,record the
formation of neoplastic cell and the general state.6 w later
unifily execute,the
tumor tissue is established light microscope blade as a routine,observe
under micro.
Results :
1. The
survey of cell transfection result 48h
after cell transfection,use Fluophot micro to observe the
transient expression of green flourescence fusion protein in
cells,as
well as calculate the transfection efficacy which is 18%,see
Fig 1.

Fig. 1 Expression
of Green fluorescent protein in MG63 Cells by transfection after
48h
2. The separation of monoplast clone
Obtain 12 lines of MG63 cell subseries which are numbered
M1-M12 respectively through limiting dilution assay.
3. The
velocity of cell electrophoresis
Process cell electrophoresis censorship on each cell
line of M1-M12, divided into highly
metastasis M8 and lowly metastasis M6,the
result see Fig 2,the electrophorogram of M6 and M8
see Fig 3。
|
|
M1
|
M2
|
M3
|
M4
|
M5
|
M6
|
M7
|
M8
|
M9
|
M10
|
M11
|
M12
|
|
t
|
11.73
|
13.02
|
15.56
|
12.32
|
14.07
|
9.69
|
11.38
|
15.64
|
11.52
|
14.08
|
12.92
|
10.62
|
Fig 2 The
electrophoresis velocity of MG63 cell substrain
<
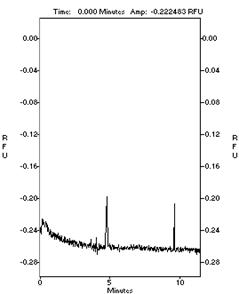
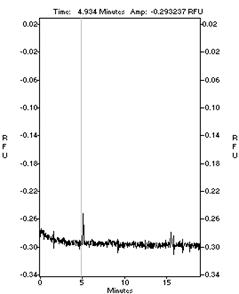
M6
M8
Fig 2 The
cell electrophoregram of M6 and M8
4. Chromosome analysis Chromosome
modal number disposition and karyotype analysis,the modal
number of M8 and M6 is centered between 61~65 separately
which are both hypotriploid ,the appearance are still
anthropo-chromatoid,the three kinds of cells chromosome
have plesiomorphism.
5. Cell morphological check
Under contrast phase microscope,cultured cell appearance
is aligned epidermically invitro,cell monolayer-poietic
has the competence of overlapping growth. Under light microscope
the cells of each group have plesiomorphism,kytoplasm is
coorespondencely less,cell heteromorphism is significant,it
is thus clear minority tumor giant cell,can be seen
caryomitosis figure accidently,cells are assumed
suffusion distribution. Under TEM the cells of every group are
plesiomorphism,there is major RI in periplast,density
is high,coloring is deep,can be seen comparatively
large mitochondria,turgescent poly-lysosome and
poly-mitochondria,the quantity of endocytoplasmic
reticulum is little,some distension,some short and
with Ves shape.Compared with M6,M8 endoplast is larger
and irregular,there is evident Ves shape protrution
on the surface of M8 cell,microvilli is long and much,there
is no marked morphological difference between M6,M8 and
M.
6. Growth curve
observation(MTT chromatometry) The
characteristics of cell proliferation 3~6d
after cell substrain postinoculation it is exponential growing,after
6d cell growth decreased,living cells decreased by the
8th day.The morphous of growth curve is basi-uniformal with
parental generational cells,M6 and M8 cell population
doubling time is 38.4h and 23.0h respectively in logathmic
growth phase.The double time of maternal plant cell is M8
32.65h. It hints M8 growing active, M6 growing
comparetively slow.
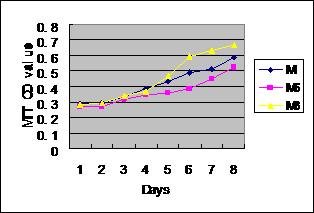
Fig. 3 Growth curve of MG63 Cells by MTT method
7. Cloning efficiency M8
plastidogenetic clone is more,
M6 plastidogenetic clone is less,presented
spindle shape just like maternal plant.
The clonies,cloning efficiency % of M6,M8 and the number of
maternal plant
|
cell subline
|
number of cells
100
50
|
cloning
efficiency/%
|
|
M6
|
18
10
|
18.7
|
|
M8
|
29
15
|
29.3﹡
|
|
M
|
23
11
|
23.7
|
*
P<0.05
(chi
square test) Marked difference in M8 vs M6
Table 1: the Potential of Forming Colonies of MG63 Cell
Lines in Soft Agar
8. The growth state of
bearing cancer in nude mice in vivo M6,M8
and maternal plant M are subsutaneously inoculated on the back
of nude mice,
3 mice have tumor formation after 5d M8 inoculation,on
15d,the
average diameter of tumor is
0.69cm
×
0.55cm
, 2 mice
have tumor formation after 8d M6 innoculation, on 15d the
average diameter of tumor is
0.29cm
×
0.77cm
, 2 mice have tumor formation after 7d M innoculation,on
15d the average diameter of tumor is
0.32cm
×
0.61cm
, execute 6 w
after operation,the
three lines have no pulmonary metastasis in anatomy. The
organization of tumor-forming is accredited caryomegaly, thick
dye,multiple mucleoli, a lot of karyokinesis phase, unclean
circumsciption between cells by HE pathological section.
Discussion :
The
infestation of malignant tumor and the regulation mechanizm of
metastatic molecule are the hot spot topics at present in the
investigation of tumor. The formation of metastasis is not only
the accomodation of tumor cells to certain organic environment
but also include primary tumor has oncocyte subseries which
possesses different metastatic potential [2],they
present heterogeneous phenomenon on the biological
characteristics. Heterogeneity of tumor is roughly displayed on
the difference of immunogenicity,growth
kinetics,metabolic
capability,karyotype,mobility,invasion,metastasis,cell
membrane receptor,the sensitivity on radiotherapy and
chemotherapy,the
foundation of heterogeneity production is due to considerable
transformed cells,it
is independent of the original of PT monoclone and polyclone.
Fidler and etc. refer the hypothesis of tumor cell heterogeneity,they
consider the metastasis of tumor is not random,there
are only very few tumor cells in primary tumor which possess the
metastatic potential can ultimately metastasize, 99% of
circulation cancer cells are going to die,only
less than 1% of tumor cells have the possibility of survival and
transfection,this
is maily decided by the existence of hyp-metastatic ability
clone cell in tumor cell colonies [3],the heterogeinicity of mono-clone
cell subseries extracted from heterogeinicity maternal cell
colony can be reduced to mn and its expression of metastatic
capability is stable,that
is extremely make for comparative experiment.So it has
significant pratical importance in the investigation of tumor
metastasis mechanism, the instruction of clinical treatment by
extraction of these cells to do the comparason of their
biological characteristic difference .
Osteosarcoma
is one of the most viscious tumor in bone tumor,it’s
difficult to cure radically and completely for its easy
metastasis and local infiltration,recently the study on the mechanizm
of osteosarcoma metastasis is more [4,5],MG63 cell line is a human
osteosarcoma cell line which possesses high metastatic potential
[6,7],the
experiment is through the method of cloning in vitro separating
two lines of clone cells which have similar modal number with
maternal line , still holding human chromatoid karyotype, M8 compare
with M6,cell
proliferation phase is shorter,cloning
efficiency is higher in soft agar,On morphosis,M8 cell
volume is larger,pheno-plasmosome
is evident under electron microscope,cell surface
vec pustute is much and manifest.
GFP
is a kind of photoprotein extracted from medusa which can erupt
green light under the optical excitation of 450~490nm blue light,can
be sighted directly under Fluophot .Because of Green Fluorescent
Protein is easily observed and detected,so it has
already become an important indication molecule in the study of
transgene. GFP conducts and actions as the tumor marker of gene
expression stably existing in cells,tracing
the expression level of fusion partner molecule and fixing
detecting environment changes or protein interactive marks. The
consequence is real and reliable and followed with great
interest by the people [1,8].This experiment is using the
characteristics of GFP gene to finish the construction of green
fluorescence protein gene expression bearer,the
bearer can use GFP as report gene to observe the expression
fixing and time
series changes of MG63 cells in animal invivo. We use EGFP as
the marker gene to transfect human osteosarcoma MG63 cell,48h
transient transfection efficacy is 18%,can stably
expressed through G418 screening to form resistance clone.
Meanwhilce
our comparason results display the integration and stable high
performance of exogenous gene GFP in MG63 cells have no marked
influence on the basic biological characteristics of MG63,growth rate,poorly
differentiated state,karyotype and the capability of
multi-directional differentiation in vivo,providing A/W for effective
utilizing GFP to progress tracing in MG63 cell in vivo.
Cell
electrophoresis behavior
is decided by cell surface structure and its functional status,after cell canceration,the
series of the changes of cell surface structure and its function
necessanly can affect its electrophoresis behavior,meanwhile can
change its invasion transfection capability, charge density on
the cell surface increased,the
repelling force between both increased which maybe promote the
abjunction of tumor cells from maternal nuclide. Because of
different cell subseries has different electrophoresis behavior,this method
can be taken as the screening route of the tumor cells which
possess different metastatic characteristics,can initially prefractionated on
tumor cells. Generally believe the cell electrophoresis rate is
increased on strong invasion and high metastatic cells. Take
cell electrophoresis rate as the chief indicator for screening
different metastatic capability tumor cell clone cell lines
[9].A great deal of experiments have shown growth advantage
possibly can amplicate cell subseries in vitro which have high
degree of malignance or gradually develop into the major
component of the whole tumor tissue,searching the difference of growth metastasis advangage has
pratical significance in the approach of tumor invasive
metastasis molecular mechanizm and treatment [10,11]. This
experiment is utilizing the correlation of cell electrophoresis
velocity with tumor metastasis potential to preliminarily screen
human osteosarcoma cell series,obtain
2 monoclone cell subseries.Among which the electrophoresis
velocity of M8 is 50% higher than M6,this demonstrates the variability of
tumor cell subseries is manifest.We observe from the experiment
the growth doubling time of M8 subseries is markly faster than
M6 subseries,this
demonstrates progressing variability analyzation by the
utilyzation of electrophoresis velocity difference on the
osteosarcoma cells which have good growth,active
functional status is feasibe,this has
established a better foundament for the further investigation of
osteosarcoma invasion metastasis mechanism.
Reference :
-
Rosochacki SJ, Matejczyk M. Green
Fluorescence Proteinasa molecular marker in microbiology. Acta
Microbiol Pol, 2002,51(3):205~216.
-
Gao Jin. The infestation and metastasis of cancer—fundament
and clinic [M].Beijin:Beijin Medical College, China Union
Medical University ,Union Publishing House,1996:2~3.
-
Fidler IJ. Tumor heterogentity and the
biology of cance rinvastion and metastasis. Cancer
Res,1978,38:2651
-
Jung SY, Kwak JO, Kim
HW, et al. Calcium sensing receptor forms complex with and is
up-regulated by caveolin
-1 in
cultured human osteosarcoma (Saos-2) cells. Exp
Mol Med. 2005 ,37(2):91-100.
-
Serra M, Reverter-Branchat G, Maurici D, et al. Analysis
of dihydrofolate reductase and reduced folate carrier gene
status in relation to methotrexate resistance in osteosarcoma
cells. Ann Oncol. 2004 Jan;15(1):151-60.
-
Rex C, Hay D, Lan Z, et al. Nuclear
Receptor Agonists as potential differentiation therapy agents
for human osterosarcoma. Clin Cancer Res 2002, 8:1288~1294.
-
Masahiko K, Noriko S, Akihiro F, et al. Laminin r2-Chain Fragment in the Circulation: A Prognostic
Indicator of Epithelial Tumor Invasion. Cancer Res,
2003,63:222~229.
-
Liu Mefang,Wang
Enduo.Green Fluorescence Protein. The development of biochemistry and biophysics,2000,27(3):238~243.
-
Gao Jin, Liu Yaqin, Han Liqun and etc..
The separation and appreciation of different metastatic
potentiality cancer cell subseries as well as the modeling of
cell clone and the application of metastatic mechanizm
investigation.China Tumor,1997,6(2),20~21
-
Calvo A, Xiao N, Kang J, et
al. Alterations
in gene expression profiles during prostate cancer progression:
functional correlations to tumorigenicity and down-regulation of
selenoprotein-P in mouse and human tumors. Cancer
Res. 2002 ,62(18):5325~5335.
-
Fan DG, Fan QY, Zhang HZ,et al. Study on
metastasis-associated gene in osteosarcoma by cDNA microarry. J
Fourth Mil Med Univ, 2003,24(9):816~819.
|






