|
J.Orthopaedics 2007;4(4)e2
index.htm
Introduction:
Despite decades of experience in fracture management, not until
recently have the biochemical markers of fracture healing been
discovered. Fibroblasts produces dermatin sulfate in the early
fracture callus (1,2,3). During the second week after a
fracture, chondroitin sulfate is expressed in large amounts by
the chondrocytes (2,4,5). However, by the third week, the
amount of proteoglycans and their aggregates decrease, and
mineralization of the fracture callus begins. Approximately 9
days after a fracture, there is an abundant expression of type
II collagen, the major structural protein of cartilage.
Chondrocytes produces chondroitin
sulfate during these first 9 days of callus formation.
By the end of the second week the events involved in the
production of cartilage switch off (1,2,3,6).
Pharmacological facilitation of the steps in callus formation
and subsequent fracture healing remains unknown.
During endochondral bone formation,
proteoglycans are expressed in the extracellular matrix of the
callus (1,2,3,6).
Recent literature has increased our understanding of the
biochemical contributors in fracture healing. After an initial
hematoma phase, local proliferation and differentiation of
inflammatory cells initially occurs. This influx facilitates
proteoglycan deposition and the formation of a cartilaginous
callus which matures through discrete stages and is ultimately
remodeled into bone (1,2,3,7,8,9,10).
Callus formation occurs in a dynamic process allowing the
healing of bone. This process involves many changes in
biochemical framework as the fracture callus evolves. The
initial hematoma provides a scaffold for the deposition of
collagens types I, II, and III, glycosaminoglycans, as well as
several proteoglycans (1,2,3,11). These proteoglycans are
expressed in the extra-cellular matrix of the callus and
comprise the main ground substance of this connective tissue
(2,3).
Heparan sulfate, dermatan sulfate and chondroitin sulfate are
three of the proteoglycans that are vital components of callus
formation in the first to second week of fracture healing
(2,3,4). As healing progresses, there is abundant expression of
type II collagen, and by day nine, it becomes the major
structural component (2,3). By the third week of callus
formation the amount of proteoglycans decreases and
mineralization continues (2,3).
Chondroitin sulfate consists of linear repeating units of D-galactosamine
and D-glucoronic acid with variable sulfation patterns as seen
in Figure 1(12).
Jackson et al in
2006 examined fracture healing using the proteoglycan heparan
sulfate (4). They found that local application of 5μg heparan
sulfate to rat femoral fractures resulted in a significant
increase in callus size, as well as increased expression of
several growth factors. They concluded that heparan sulfate had
anabolic potential and may be a potential candidate therapy for
enhancing bone repair.
Several studies have used chondroitin sulfate orally to examine
its effect on cartilage (12,13,14,15,16). Oral supplementation
reduces the degradation of cartilage matrix components,
specifically collagen II, glycosaminoglycan and other
proteoglycans. These studies have also suggested that the oral
administration of chondroitin sulfate is safe
(12,13,14,15,16,17,18,19).
Rammelt et. al. has shown that chondroitin sulfate can
successfully facilitate bone healing when implants are coated
with chondroitin sulfate (11). In this study, the hypothesis
was tested that early administration of oral chondroitin sulfate
will have similar effects on bone fracture healing and callus
formation as the local direct application of chondroitin
sulfate.
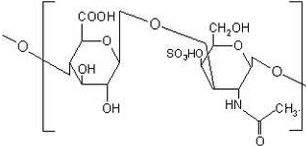
Figure 1: Chondroitin Sulfate Molecule
Material and Methods :
Experimental Design and Surgical Procedures:
Eighteen male Sprague-Dawley rats (mean weight 300g) rats were
randomized into six groups of three animals and anesthetized
using an intraperitoneal injection of 60mg/kg Ketamine and
10mg/kg Xylazine. For each rat, the fur of the left knee was
removed and the skin was sterilized using an iodine preparation.
A 5mm midline longitudinal incision was made (Figure 2). Using a
standard medial parapatellar approach, the anterior
intercondylar notch was appreciated. A 1.6mm Kirschner-wire was
inserted manually into the femoral medullary canal until the
canal was filled; the distal end of the wire was cut to sit
flush with the knee (Figure 3). After insertion, the soft
tissues were re-approximated and the incision was closed with a
simple interrupted 4-0 Monocryl suture.
Figure 2: Longitudinal incision for dissection over knee.
Figure 3: Intra-Medullary insertion of 1.6 mm K-Wire.
While still anesthetized, a blunt guillotine-like blade device
was used to generate a transverse mid-femoral closed fracture.
Radiographs were immediately taken to confirm the fracture and
confirm the intramedullary placement of the Kirschner-wire
(Figure 4).

Figure 4: Post guillotine fracture pattern.
The rats were numbered 1-18 and individually housed. Daily
buprenorphine 0.1ml was injected subcutaneously for pain control
until no signs of pain were appreciated; normal activity was
resumed within a few days.
The eighteen rats were initially separated into experimental
(#1-9) and control (#10-18) groups. These were further
subdivided into three groups of three rats each to be euthanized
at 1 week (Group A), 4 weeks (Group B), and 5 weeks (Group C)
time points. All experimental rats were dosed once daily with a
solution of 7mg Chondrotin Sulfate in 1mL deionized water
delivered via oral gavage tubes. The animals were dosed for
nine days following the procedure (except for Group A animals
which were sacrificed at 1 week.).
Animals were euthanized at their respective time points by CO2
asphyxiation and the left legs were disarticulated at the hip
joint. After careful dissection of the surrounding soft tissue,
the femurs were removed (Figure 5). The Kirschner-wires were
removed and the femurs were placed in a 70% formalin solution at
4°C.
Figure 5: The soft tissue was removed prior to amputation
of the limb.
MicroCT Scanning
To accurately describe the morphology of the healing fracture
site, the diaphysis of each femur was scanned via micro-computed
tomography (MicroCT40, Scanco Medical, SUI) at a resolution of
15 microns (70KV, 114uA). Gaussian filtering removed noise from
the images and global thresholding segmented mineralized tissue
from soft tissues and bone marrow. Within 216 slices of the mid-diaphysis,
the following morphometric parameters were determined via
software provided by the manufacturer: cross-sectional bone
area, area of the periosteal envelope, area of the endocortical
envelope, moments of inertia, transcortical thickness, cortical
porosity, and the volumetric density of the mineralized tissue
(Figure 6).
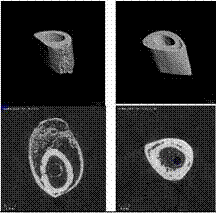
Figure 6: Micro-CT images.
Post Radiographs
Following the CT scans, the femurs were arranged on an 11x17
large radiographic cassette and anterior-posterior radiographs
were taken at a distance of 100cm (Figure 7). Radiographs were
evaluated by a senior orthopaedic surgical resident to describe
callus formation. Using
picture archiving and communication systems (PACS) computer
imaging software we were able to quantify the callus formation.
The widest transverse width and the longest longitudinal length
were measured. A quantitative measure of the callus was
calculated.
Figure 7: Radiograph of rat femurs after amputation and
removal of the K-Wire.
Histology
The rat femurs were decalcified in 5% formic acid and saturated
with ammonium oxalate and agitated for 24 hours. After
decalcification, the tissues were prepared for paraffin
embedding. The free water was removed from the tissue with
alcohol as a dehydrating agent. Xylene was used to remove the
alcohol, which is not directly miscible with paraffin. The
tissue was then submerged into melted paraffin on a tissue
processing machine.
The specimens were kept in the VIP Surgical Processor program
Overnight cycle. The 14 different stations contained the
solutions of 10% formalin, 60% ethanol, 95% ethanol, 100%
ethanol, xylene, and paraffin. Paraffin blocks were made next;
this involved enclosing the tissue in the infiltration medium
for processing allowing the medium to solidify. The final slides
were made by the removing sections of uniform thickness using
the microtome knife.
A
drop of synthetic resin was used to remove the excess xylene.
This formed a mounting medium allowing the slides to be stained.
The slides were initially stained with hematoxylin
and eosin. Lastly, the slides were stained with Sfog to
view any cartilage formation.
A board certified blinded Pathologist reviewed all of the
slides and gave mathematical percentages to granulation tissue,
cartilaginous callus, and bony callus (Figure 8).

Figure 8:
Experimental rat from group A showing premature healing.
Statistics:
Statistical evaluation was performed using Microsoft Excel for
Windows. The significance level was set to 0.05 and all T-tests
were two-sided. P-values were not adjusted for multiple
testing.
Results :
The body weights of the rats between the two groups did not vary
significantly. Of the 18 rats, three of the rats, all in the
control group, died prematurely and were excluded from the
study; (two had penetration of intra-medullary rod through the
perineum, one was overdosed on anesthesia). Two of the rats
from control group C were immediately replaced by control group
A rats to maintain a similar quantity in each five week time
frame.
During tissue processing, one of the femurs in the experimental
group broke at the callus and was excluded from the analysis.
Consequently we were able to fully analyze 14 (78%) of the
initial 18 rats.
We analyzed eight experimental rats, three that were euthanized
at 1 week (Group A; rats number 1,2,4), two were euthanized at 4
weeks (Group B; rats 5,6), and three that were euthanized at 5
weeks (Group C; rats 7,8,9). The control group was left with
six rats, one was euthanized at 1 week (Group A; rats number
10), two were euthanized at 4 weeks (Group B; rats 11,13), and
three that were euthanized at 5 weeks (Group C; rats 14,15,18).
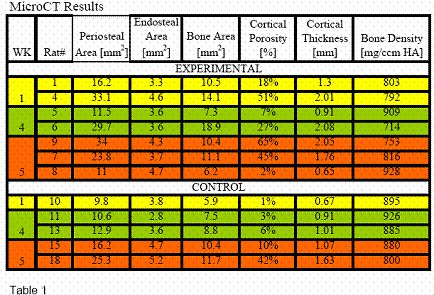
MicroCT analyses showed that, one week after fracture, the
amount of mineralized bone present in the mid-diaphysis was
two-fold greater in the experimental than in the control bones
(Table 1). This difference was entirely accounted for by the
difference in periosteal area (152% greater) while there was no
difference in the endosteal area. Because of the much greater
callus in experimental bones, cortical thickness was also 2.5
fold greater and the relative amount of cortical porosity had
increased by an order of magnitude. Tissue mineral density, an
indicator of the tissue mineralization was similar between the
groups. However, with the number of rats in this study,
statistical significance was not obtained with the MicroCt
results.
When bones from the 4wk and 5wk time periods were pooled, the
periosteal area of experimental rats was still 36% greater than
that of control rats. Similar to the one week time point, there
was no difference in the endosteal area, indicating that the
treatment effect was confined to the fracture callus. The
amount of bone present in the diaphysis was 12% greater,
accompanied by a two-fold greater cortical porosity and a 30%
greater transcortical thickness. The similarity in the density
of the mineralized matrix suggested that the treatment had no
effect on tissue mineralization. Again, P values were not
significant using the student T-test.
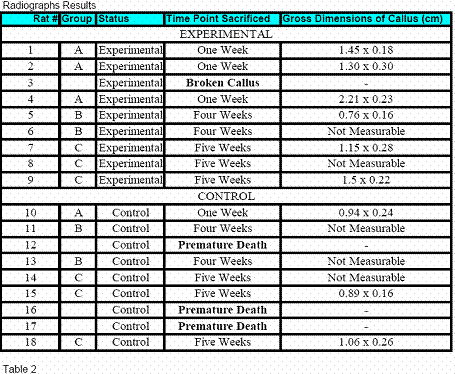
Radiographs were evaluated by a senior orthopaedic surgical
resident to evaluate callus formation. Using
picture archiving and
communication systems (PACS) computer imaging software, we were
able to quantify the callus formation. The widest transverse
width and the longest longitudinal length were measured (Table
2).
Although statistical significance was also not obtained with
radiographic analysis, there was an obvious trend towards more
robust callus in the experimental group. Acknowledging the
small sample size, no callus was appreciated in 2 (25%) of the
experimental group, and 3 (50%) of the control group.
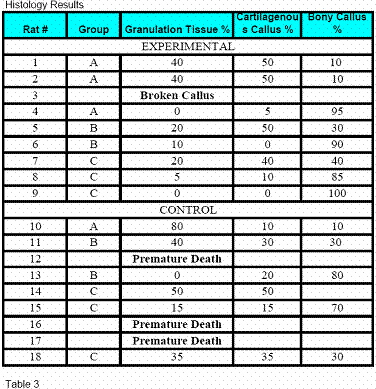
A
board certified Pathologist evaluated the histological slides.
He was blinded to the experimental and control groups. The hematoxylin
and eosin stained sections combined with the Sfog stain
facilitated in the elucidation of granulation tissue,
fibrocartilagenous and new bone formation (Table 3).
Rat bone number 2 in the
experimental group A had significant amounts of necrotic bone
and was the most premature of all the slides (Figure 8). On the
other hand, experimental rats from groups B and C (rat number
4,6,9) showed significant advancement in fracture healing with
rat number 4 in experimental group C showing complete healing
and signs of marrow regeneration (Figure 9). These patterns of
fracture healing were also evident in the MicroCT results with
the most pronounced periosteal bone formation. The plain
radiographs, although one dimensional, also had a significant
increase in callus formation compared to the other specimens.
At the same time points, there was a trend towards advanced
histological progression of callus in the experimental group
(Figures 10, 11).

Figure 9:
Experimental rat from group C showing almost complete healing.
 
Figure 10, 11:
Trend towards histological progression of callus in experimental
group.
Discussion:
This pilot study demonstrated that
supplementation of oral proteoglycans to acute fractures may
facilitate bone healing. Chondroitin sulfate is a proteoglycan
that has been found to be a key biochemical contributor in the
early phases of bone healing. The initial fracture
hematoma is organized by the infiltration of local inflammatory
cells and subsequent proteoglycan deposition. Further remodeling
yields a cartilaginous callus which matures through discrete
stages and is ultimately remodeled into bone. (2,6). In this
study, chondroitin sulfate given during the initial hematoma
phase showed differences in the periosteal area of experimental
rats indicating that the treatment effect was confined to the
fracture callus. Additionally, the histology demonstrated
advances in marrow formation and bony callus compared to the
control group. However, P value of <0.5 could not be achieved
with the small sample size of this pilot study.
This study showed that a single oral daily dose of chondroitin
sulfate increased the callus size by 152% within the first week
and over a period of 5 weeks the mean callus size was over 36%
larger than the control group. The chondroitin sulfate did not
increase the rate of endochondral healing as evidenced by the
same proportions of endosteal area in the experimental as well
as the control group (Table 1). The increase in bone content
suggests that more bone tissue formed through intramembranous
ossification rather than endochondral ossification.
Heparan sulfate, dermatan sulfate and chondroitin sulfate are
three of the proteoglycans that are vital components of callus
formation in the first to second week of fracture healing. Song
et. al. showed that heparin sulfate and chondroitin sulfate
expression was generally found to increase in the days
immediately following injury, reaching peak expression two weeks
post-surgery (3). These authors suggested the possibility of
using exogenous proteoglycans as an adjunct to fracture healing
(3).
In 1962, Burger et. al. documented accelerated new bone
formation using chondroitin sulfate in rat cranial bone defects
(17). In 1962 they published a follow up study comparing their
study arm of demineralized bone and chondroitin sulfate compared
to a control group without chondroitin sulfate in Wistar albino
rat’s cranium. Thirty three percent less time (six weeks versus
nine weeks) was required in the experimental group to achieve
maximal healing (17).
Jackson et. al. in 2006 examined the augmentation of fracture
healing using an exogenous proteoglycan (4). They found that
local application of 5μg heparan sulfate to rat femoral
fractures resulted in a significant increase in callus size, as
well as increased expression of several growth factors. They
concluded that heparan sulfate had anabolic potential and may be
a potential candidate therapy for enhancing bone repair.
Although effective, heparan sulfate is not routinely used, nor
readily available for oral administration in humans. It would be
difficult to perform this in vivo to humans with acute
fractures. Oral chondroitin sulfate has been proven to be safe
and may be effective in augmenting fracture healing if given
during the hematoma phase of bone injury.
A pilot study has inherent limitations. Due to the small sample
size in each group, statistical significance could not be
obtained for each criteria measured. Radiographs demonstrated
to be the least helpful tool in defining callus formation, and
have been confirmed in the literature to be the least sensitive
tool in addressing fracture healing in rats (20). Three
dimensional imaging and histology have been promoted in the
literature as more sensitive tools and was found to be useful in
this pilot study (20). There was a trend towards increased
callus formation in all of the experimental arms, most notably
with the micro CAT scan. The pathology results also
demonstrated a strong correlation between chondroitin sulfate
and increased callus formation.
The pharmacologic response to chondroitin sulfate in patients
with knee osteoarthritis was analyzed (21,22). It was
discovered that chondroitin sulfate is a slow acting drug. This
study showed that pharmacologic response increases as a function
of time until it reaches the maximal effect, even after
cessation of treatment (21,22). We believe that by taking
advantage of the long acting effects of chondroitin sulfate, it
could be used as a safe and effective tool to facilitate bone
remodeling, specifically in bones notorious for non-union
(23,24,25,26,27).
To our knowledge, the mechanism of bone repair by chondroitin
sulfate is yet unclear. Based on the results of our study we
would suggest that chondroitin sulfate has the potential to
enhance callus formation. This study provides a framework for
future studies to investigate oral supplementation for the
facilitation of fracture healing.
In addition, chondroitin sulfate chains often contain multiple
protein binding sites, the particular sulfation pattern of
bone-specific chondroitin sulfate, and its resultant growth
factor-binding capabilities need to be investigated with further
research to determine the best combination for enhancing bone
repair.
Reference :
1. Aro HT, Wippermann BW, Hodgson SF, Chao EY. Internal
remodeling of periosteal new bone during fracture healing.
Journal of Orthopaedic Research:1990 Mar;8(2):238-46.
2. Joseph A. Buckwalter, MD, Thomas A. Einhorn, MD, Sheldon R.
Simon, MD, Editors. Orthopaedic Basic Science: Biology and
Biomechanics of the Musculoskeletal System, 2nd Edition. Chapter
14. 2000.
3. Song SJ Hutmacher D Cool S.M Nurcombe V Gene Temporal
expression of proteoglycans in eth rat limb during bone healing
379 (2006) 92-100.
4. Jackson R.A., McDonald M, Nurcombe V, Little D., Cool S., et
al. The Use of Heparan Sulfate to Augment Fracture Repair in a
Rat Fracture Model. J of Ortho Res. 2006 Apr;24(4):636-44.
5. Page M, Ashhurst DE. The effects of mechanical stability on
the macromolecules of the connective tissue matrices produced
during fracture healing. II. The glycosaminoglycans. Histochem
Journal. 1987 Jan;19(1):39-61.
6. Opolka A, Ratzinger S, Schubert T, Spiegel H U, Grifka J,
Bruckner P, Probst A, Grassel S. Collagen IX is indispensable
for timely maturation of the cartilage during fracture repair in
mice. Matrix Biol. 2007 Mar;26(2):85-95.
7. Chao EY, Inoue N. Biophysical stimulation of bone fracture
repair, regeneration and remodelling. European Cells and
Materials. 2003 Dec 31;6:72-84.
8. Keskin DS, Tezcaner A, Korkusuz P, Korkusuz F, Hasirci V.
Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2
delivery system for bone repair. Biomaterials. 2005
Jun;26(18):4023-34.
9. Schmidt AH, Finkemeier CG, Tornetta P 3rd. Treatment of
closed tibial fractures. JBJS: Instructional Course Lecture.
2003;52:352-368.
10. Zou X.H, Foong W.C, Cao T., Bay B.H., Ouyang H.W. and Yip
G.W. Chondroitin Sulfate in Palatal Wound Healing J Dent Res
83(11) 880-885, 2004.
11. Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H,
Schneiders W. Coating of titanium implants with collagen, RGD
peptide and chondroitin sulfate. Biomaterials. 2006 Nov; 27(32):
5561-71.
12. Richy F, et al. Structural and symptomatic efficacy of
glucosamine and chondroitin in knee osteoarthritis: a
comprehensive meta-analysis. Arch Intern Med.
2003;163:1514-1522.
13. Clegg DO, et al. Glucosamine, chondroitin sulfate, and the
two in combination for painful knee osteoarthritis. N Engl J
Med. 2006;354:795-808.
14. Michel BA, et al. Chondroitins 4 and 6 sulfate in
osteoarthritis of the knee: A randomized, controlled trial.
Arthritis Rheum. 2005;52:779-786.
15. Uebelhart D, Thonar EJ, Delmas PD, Chantraine A, Vignon E.
Effects of oral chondroitin sulfate on the progression of knee
osteoarthritis: a pilot study. Osteoarthritis Cartilage. 1998;6
Suppl A:6-13.
16. Uebelhart D, Malaise M, Marcolongo R, DeVathaire F, Piperno
M, Mailleux E, Fioravanti A, Matoso L, Vignon E. Intermittent
treatment of knee osteoarthritis with oral chondroitin sulfate:
a one-year, randomized, double-blind, multicenter study versus
placebo. Osteoarthritis Cartilage. 2004 Apr;12(4):269-76.
17. Burger M, Sherman B, Sobel A. Observations of the influence
of chondroitin sulfate on the rate of bone repair. Journal of
Bone and Joint Surgery, Br. 1962;44(3):675-687.
18. Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG,
Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M,
McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G,
Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis:
new insights. Part 1: the disease and its risk factors. Ann
Intern Med. 2000 Oct 17;133(8):635-46.
19. Michel BA, Stucki G, Frey D, De Vathaire F, Vignon E,
Bruehlmann P, Uebelhart D. Chondroitins 4 and 6 sulfate in
osteoarthritis of the knee: a randomized, controlled trial.
Arthritis Rheum. 2005 Mar;52(3):779-86.
20. Aro HT, Wipperman BW, Hodgson SF, Wahner HW, Lewallen DG,
Chao EY. Prediction of properties of fracture callus by
measurement of mineral density using micro-bone densitometry. J
Bone Joint Surg 1989;71:1020-1030.
21. Du Souich P, Vergés Josep. Simple approach to predict the
maximal effect elicited by a drug when plasma concentrations are
not available or are dissociated from the effect, as illustrated
with chondroitin sulfate data. Clinical Pharmacology &
Therapeutics (2001);70:5–9.
22. Verges J, Souich PD. Simple approach to predict the maximal
effect elicited by a drug when plasma concentrations are not
available or are dissociated from the effect, as illustrated
with chondroitin sulfate data. Clinical Pharmacology &
Therapeutics, 70(1), July 2001, 5-9.
23. Bone LB, Sucato D, Stegemann PM, Rohrbacher BJ. Displaced
isolated fractures of the tibial shaft treated with either a
cast or intramedullary nailing. A randomized prospective trial.
Journal of Bone and Joint Surgery, Am. 1997;79:1336-41.
24. Hak DJ, Lee SS, Goulet JA. Success of exchange reamed
intramedullary nailing for femoral shaft nonunion or delayed
union. Journal of Orthopaedic Trauma. 2000 Mar-Apr;14(3):178-82.
25. Khanal GP, Garg M, Singh GK. A prospective randomized trial
of percutaneous marrow injection in a series of closed fresh
tibial fractures. International Orthopaedics. 2004
Jun;28(3):167-70. Epub 2004 Mar 9.
26. Marsh D. Concepts of fracture union, delayed union, and
nonunion. Clin Orthop Relat Res. 1998 Oct;(355 Suppl):S22-30.
27. Sarmiento A, Sharpe FE, Ebramzadeh E, Normand P, Shankwiler
J. Factors influencing the outcome of closed tibial fractures
treated with functional bracing. Clinical Orthopaedics.
1995;315:8-24.
|
















