|
J.Orthopaedics 2007;4(1)e15
Introduction:
Gas gangrene is an extremely severe surgical infection with high
mortality (1-6). It is a fulminant necrotizing soft tissue
infection caused by anaerobic bacteriae belonging to genus
Clostridium which are Gram positive, cylindrical in shape
bacilli and produce endospores (1,2,3,7). Pathogenic Clostridia
produce toxins developing tissue necrosis (1,7). The main
pathogens in gas gangrene are: C. perfringens type A (85- 90%)
(1,7,8) and C. septicum, C. novyi, C. histolyticum, C. sordelli,
C. fallax, C. carnis (1,3,4). Clostridial infections are usually
polymicrobial (1).
Representatives of the genus Clostridium are found mainly as
endospores in soil, dust and constitute a common component of
the bowel flora in humans and animals (C. perfringens) or female
genitourinary tract (mainly C. septicum) (1,2,3). In general
Clostridia are widely distributed in nature (1,2).
In this paper we present two patients with severe gas gangrene
syndromes of a completely different origin in order to
present that patients following trauma may develop infections
originating both from their own bacterial flora and from an
external source (1,2,5). Clostridial infections in these
patients were confirmed by positive cultures.
Case reports:
Case 1.
A 65 old male, was admitted to our Department following severe
shotgun injury of the chest and left shoulder girdle. He shot
himself accidentally during the poaching expedition while using
a sewed-off shotgun. At the admission he was under the influence
of alcohole (serum ethanole level 2.29 0/00). The patient was in
a profound traumatic shock, with poor respiration, unconscious.
Resuscitation procedures were initiated and after performing
X-rays multifragmental left scapula fracture, multiple left rib
fractures, left haemopneumothorax, vast subcutaneous thoracic
emphysema were diagnosed. It was decided to operate on the
patient. A left latero-posterior thoracotomy was performed,
vast pleural haematoma was evacuated and lacerations of the lung
tissue were sutured; pleural suction drainage was installed.
After the surgery the patient was admitted to the ICU, intubated
with controlled mechanical ventilation. Wide-spectrum
empirical antibioticotics was administered-
piperacillin+tazobactam (Tazocin) 3x4,5/day i.v. During three
postoperative days the patient was stable- chest
X-ray examination showed parenchymal densities in the left lung,
bronchofiberoaspiration (BFA) was performed, pleural cavity
drainage reached 150-200 ml/day. The patient’s temperature was
mildly elevated- 37oC. On the fourth day a serosanguineous
exsudate from the postoperative wound was observed; surrounding
tissues were mildly reddened, oedematous. Subcutaneous
crepitations were noted. An urgent surgical intervention was
instituted with the vast excision of necrotic tissues (Fig.1).
Tissue and fluid specimens were microbiologically examined and
cultured. Direct microscopic examination showed large number of
Gram positive cylindrical- and spheric- shaped forms. Cultures
revealed multibacterial growth- C. perfringens, Staphylococcus
aureus and Enterococcus sp. C. perfringens strain was
identified as type A (alpha, theta and kappa toxins producing).
The patient was transferred to the Hyperbaric Medicine
Department (head dr Z.Sićko). He stayed there for 8 days,
receiving 15 sessions of hyperbaric oxygen therapy (HBO).
Antibioticotherapy was modified- the patient was given
intravenously penicillin (3x10 mln u./day) and metronidazole
(3x0.5/day i.v.). Besides he was given a complete parenteral
alimentation. The wound dressings were changed every day with
further surgical debridement; dissinfectants (Octenisept,
Hibitane) were used locally. Further microbiological procedures
showed no anaerobic growth; hospital multi-resistant strains of
Enterococcus faecium and Acinetobacter baumannii were cultured.
These findings forced further antibioticotherapy modifications
due to pathogens susceptibility- vancomycin (2x1.0/day i.v.) and
netilmycin (3x0,05/day i.v.) instead of penicillin; with further
therapy with metronidazole. After 8 days the patient left the
ICU. Another surgical excision of necrotic tissues from the left
axillar region was performed. Two consecutive skin
transplantations were also performed. Full rehabilitation
program was introduced. After 67 days of hospitalisation the
patient went home in good condition.
Case 2.
A 60 year-old male was admitted to our Department for an
elective operation in order to remove osteosynthesis
implants. The patient underwent osteosynthesis a year before
because of pertrochanteric right femur fracture. Complete bone
union of the fractured bone was achieved. The patient was
homeless, suffered from gastric and duodenal ulcerative disease.
Surgical removal of an angled plate from the femur was
performed. On the third postoperative day the wound region
became painful, with pale, oedematous marigins, and
serosanguineous exsudate. The wound was opened in the upper part
and a drain was installed. The fluid from the wound was
taken for microbiological examination. Empirical
antibioticotherapy was initiated- ampicillin+sulbactam (Unasyn)
2x1,5/day i.v. No disorders of consciousness were present.
On the fifth postoperative day positive bacterial cultures
revealed- C. perfringens, C. bifermentans. The wound was widely
reopened (Fig. 2); necrotic tissues, with cooked-like muscles
were extensively excised. Microbiological examinations were
repeated revealing the same bacteriae- C. perfringens, C.
bifermentans. C. perfringens strain was identified as type
A (alpha, theta and kappa toxins positive). The patient was
transferred to the Hyperbaric Medicine Department. He was
treated with HBO for 3 days; 5 sessions of HBO. The patient was
given intravenously penicillin (3x8 mln u./day) and
metronidazole (3x0.5/day i.v.). The wound dressings were changed
every day; necrotic tissues were removed; dissinfectants (Octenisept,
Hibitane, Betadine) were used locally. A significant local
improvement was observed; on the 18-th day after the operation
the wound was resutured. Rehabilitation was initiated. On the
40-th postoperative day the patient was excribed home in good
condition.
Both patients’ C. perfringens isolates were cultured on the
Columbia Sheep Blood Agar anaerobically.
They were further identified by a classical method and VITEK ANI
cards (bioMerieux). Patients’ cultured strains from the
genus Clostridium were isolated and kept in lyophilised at
the Department of Sera and Vaccines Evaluation at the National
Institute of Hygiene in Warsaw, Poland.
C. perfringens strains were identified by Gram staining, urease
and lecithinase assays, and other biochemical tests (9). The
biotype of C. perfringens strains was determined by
seroneutralization of lethality using intravenous injection in
mice at the time of strain identification during (9). Multiplex
PCR to detect C. perfringens were performed with primers
designed for plc, cpb1, etx, and iap genes fragments. Primers
sequence and PCR conditions were previously described (9).
Classical biotyping of analysed C. perfringens collection,
performed by the seroneutralisation of lethality by intravenous
injection in mice revealed that both C. perfringens strains were
of biotype A ( alpha, theta and kappa toxins positive). Similar
data of toxin type determination were obtained by multiplex PCR
for both C. perfringens strains, as amplification was successful
for 402 bp product for cpa1/cpa2 primers designed for plc gene,
and there were no products for cpb3/cpb4, cpe1/cpe2, and
cpi1/cpi2 primers designed for detection of cpb1, etx, and iap
genes, respectively.
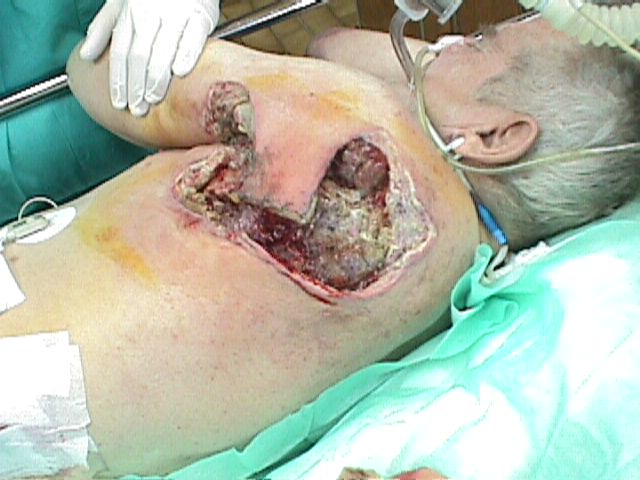 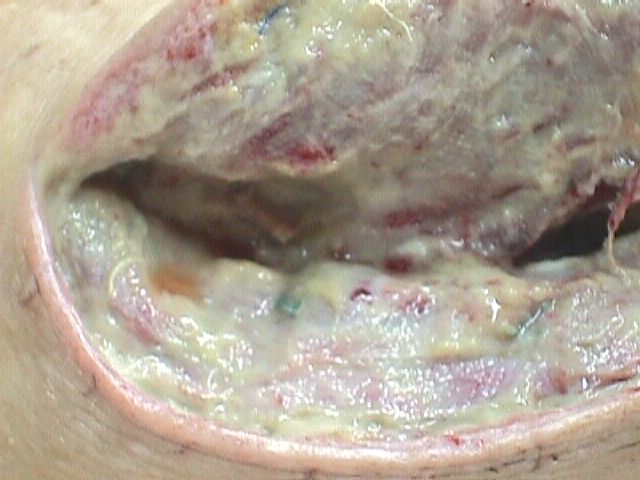
Fig. 1:
The debrided area of the wound revealing devitalized muscles and
tissue.
Fig. 2:
Open wound with characteristic pale disintegrating muscles and
exsudate.
Discussion :
During the World War I in the Northern Europe the incidence of
gas gangrene was 12% with the mortality of 25% (1). The
introduction of early wound excision resulted in a significant
decrease of gas gangrene down to 1% by the end of WW I (1) .
During the WWII the incidence of this infection among British
soldiers ranged from 0.3 to 0.8 %; the incidence among American
troops varied from 0 to 4.5 % (1) with 5% rate in severe wounds
(2). Nowadays, the morbidity is assumed to be less than 0.08 %,
with no mortality(1).
In the 1970s, according to the British and American authors the
incidence of gas gangrene in civilian practice varied from 0.1
to 1 per million population per year and is connected mainly
with trauma (1). According to Abella BS, Kuchnic P,
Hiraoka T et al. about 1000 cases of gas gangrene are recorded
each year in the USA (2). There are no precise epidemiologic
data concerning this issue in Poland .
It is estimated that about 50% of clostridial infections are
posttraumatic, 35% develop in vast surgical wounds, 15% are
spontaneous, atraumatic or metastatic (2).
In the above described cases the most severe type of clostridial
infection- i.e. myonecrosis with extensive muscle involvement
and severe toxemia was observed. In such cases clinical symptoms
of gas gangrene may appear as early as 6-8 hours from the onset
(1,7). The linear progress of the inflammation may be as fast as
several cm per hour (1,2,7). The incidence of septic shock and
multiorgan failure (MOF) in such cases is estimated at 50%, and
mortality rate rises up to 40% (7).
Clostridium genus multiply and produce toxins responsible for
the clinical symptoms of the disease only when the
oxidation-reduction potential of the tissues is below + 74 mV
(normal values are from +126 to +246 mV) (1). Such conditions
occur as a consequence of local or systemic tissue anoxia.(i.e
caused by posttraumatic, cardiogenic or hypovolemic shock)
(1,2). Gunshot wounds cause extensive tissue damage by temporary
and permanent cavities and disturb local tissue perfusion (1).
Crush injuries of limbs are connected with significant blood
loss and poor perfusion (1). Inadequacies in management of limb
injuries i.e application of tourniquets and too tight plasters,
missed diagnosis of vessels injuries cause similar conditions
(1). Any damage involving the skin is of great importance as
potential invasion gates of the infection (1,2,4,5). Metal
implants might carry the pathogens (1,6). There are more sources
of contamination: missiles and their fragments, pieces of
clothing and tissues (1,6). Soil that contaminates the wound
usually contains large numbers of aerobic bacteria like
Escherichia coli and Proteus sp (1). They contribute to the
initiation of anaerobic bacterial growth by decreasing the
oxidation-reduction potential. This enables the spores of
Clostridium to change into vegetative forms, multiply and
produce toxins responsible for clostridial infection symptoms
(1,5,7). This course of events is typical for posttraumatic gas
gangrene. Gas gangrene may also develop after elective surgery,
i.e amputation for obliterative arterial disease, surgical
stabilisation of lower limb fractures, gastrointestinal surgery
especially including bowel and biliary tract, artificial
abortions (1,2,5). Microorganisms resposible for the infection
in these cases come from the endogenous flora of the buttock and
tight skin and mucosa (1-6). The application of bloodless field
techniques, insufficient haemostasis, excessive use of
diathermy, aggressive operative procedures enable these bacteria
to infiltrate the damaged tissues (1,5).
Clinical symptoms of gas gangrene may coexist with those
induced by the trauma like: contusions, abrasions, fractures and
tight plasters. A suprisingly strong pain results from the
tension of the affected tissue (1,2,4). Other specific symptoms
are: crepitus (when the infection begins in deep layers of the
wound it is not always present), typical unpleasant, foul smell,
oedema, erythema, blisters filled with serous or saiguinoserous
fluid (1,2,5). The margins of the wound are necrotic and its
inner parts reveal pale, disintegrated muscles (1). The arterial
pulse may be impalpable and the rigidity of muscles may be
observed.
General symptoms depend on the level of toxemia, including
symptoms of toxic shock and multiorgan failure (1,2,3,6).
Frequent pulse, inadequate to the mild rise of body temperature
is one of the early ones (1,2). Disorders of the patients’
consciousness are manifestation of process affecting the central
nervous system- encephalopathy(5). When they appear at the onset
of the disease, the prognosis is usually poor. Encephalopathy is
a result of damage of cerebral blood vessels by clostridial
toxins (7,8). Further progress of the disease may result in
significant downfall of arterial blood pressure, eventually
resulting in shock (2,3,5,6). Body temperature rises
moderately, and the lack of fever may suggest poor prognosis.
Haemolytic anaemia, throbocythopenia and jaundice, acute renal
failure may be observed (1,2,5,6). Gas gangrene may lead to
clostridial sepsis and haematogenic spread of the infection
resulting in secondary focus of infection (1,3).
“Key toxins” are: alpha toxin and theta toxin (1,7,8). Alpha
toxin possesses activities of lecithinase (PLC) and
haemolysine (1,7,8). High concentrations of alpha toxin
are required to cause clinical symptoms of gas gangrene (2,7,8).
Alpha toxin causes damage, lysis of cell membranes which
has a lethal effect to various tissues i.e. haemolysis, lysis
and aggregation of platellets, lesion of leucocytes and
endothelial cells, creation of conglomerates of fibrin,
leucocytes, platelets which obliterate blood vessels,
lesion of myocardium cells resulting in arrythmias, bradycardia,
hypotension, cardiogenic shock (7,8). Alpha toxin has got also
dermonecrotic properties (7). Alpha toxin inhibits
functions of neutrophils, which are unable to penetrate the
infected tissues (leucostasis) (2,7,8). It is assumed that alpha
toxins pathogenicity is bound with the arachidonic acid cascade
(7,8). Theta toxin is called a lethal haemolysin or
perfringolysin O. It is responsible for the necrosis of tissues.
It also increases permeability of blood vessels and is
cardiotoxic (7,8). The synergic interactions of both toxins is
of great importance, as they decrease tissues perfusion by
damaging capillaries (7,8).
While performing bacteriological diagnosis of gas gangrene it is
essential to obtain biological materials from the “borderline”
of the infected area (fragments of tissues, discharge) (1,4,6).
Smears taken from the wound are not recommended. The biological
samples undergoes direct examination by Gram
staining(1,2,4,5,6). Members of genus Clostridium present
themselves in microscopic examination as cylindrically shaped,
gram-positive forms(1,2,4). A positive culture is the final
confirmation of the diagnosis (1,4,5). Histopatologic
examinations of the possibly infected tissue might be helpful in
establishing quick diagnosis (1,2).
The progress of gas gangrene is rapid. Prognosis therefore
relies on the time of diagnosis. Two elements are necessary to
establish proper diagnosis: the typical clinical course and the
presence of cylindrical, gram-positive forms in direct
microscopic examination (4,5).(2,4). It involved radical
excision of all the affected tissues and amputation procedures
(2,4,5). The results of amputations were often unsatisfactory as
they were not radical enough and patients required higher limb
reamputations. Other historical methods of treatment included
dressing the wound using H2O2, chloramine and other oxygen
“carriers” combined with wide incisions of the affected soft
tissues. Deep intramuscular injections of pure oxygen were also
performed. These methods were generally ineffective; they could
not stop progress of the disease. Antitoxin also did not
prove to be a satisfactory treatment of gas gangrene. HBO was
introduced in the mid 70’s (1,10). Nowadays it is generally
accepted as the treatment of choice in anaerobic infections,
especially in cases of gas gangrene (2,4,5,10). It decreases
mortality and shortens the time of treatment; it inhibits the
production of toxins and significantly improves the oxygenation
of the damaged tissues and the cytotoxic abilities of
phagocytes (10).
Successful treatment of gas gangene is always a combined one
(4,5,10). HBO therapy should be completed by surgical
procedures, empirical antimicrobial therapy and application of
antiseptics. Antiseptics effective against Clostridium spp,
including spores are: 0,1% Octenisept, 10% Betadine, 5%
Hibitane (4). Based on our clinical experience we prefer to use
Octenisept because of its high antibacterial efficacy, good
tolerance and safety.
Clostridia associated with wound infections are susceptible to
several antimicrobial agents: penicilin (2,5) combined with
lincosamides i.e clindamycin or metronidazole(5,6).
However surgery still plays an essential part in the proper
management of gas gangrene. Nowadays the operations are not as
radical as they used to be in the past. They include incisions
widening the wounds, relieving tension of the soft tissues
(2,5). The necrotic tissues are removed but the excision should
not be too radical because they undergo a demarcation during
hyperbaric oxygen therapy (5,10). Lately amputations in
posttraumatic cases are rarely performed (5).
A detailed knowledge of the clinical course, pathophysiology,
diagnostic and up to date methods of treatment of gas gangrene
are essential for surgeons and emergency medicine specialists.
In cases of this infection rapid and adequate management usually
results in saving patient’s life and allows to avoid serious
body disability. All cases with surgical soft tissue infections
should be monitored microbiologically (5).
It is worth considering that patients planned for elective
surgical procedures should have a rectal tamponage with
dissinfectants (Octenisept, Betadine, Hibitane) in prevention of
endogenous infections of the opperative field.
Proper management of gas gangrene should be based on close
cooperation between a surgical staff and a microbiological
laboratory experienced in problems of clostridial infections.
Reference :
- Sussmann M, Boriello SP, Taylor DJ. Gas gangrene and other
clostridial infections. In: Collier L, Balows A, Sussman M,
eds. Topley and Wilsons’ Microbiology and Microbial
infections, Ninth edition. London, Arnold; 1998:669-684.
- Abella BS, Kuchnic P, Hiraoka T et al. Atraumatic
clostridial myonecrosis: case report and literature review. J
Emerg Med. 2003;4:401-405.
- Chew SSB, Lubowski DZ. Clostridium septicum and
malignancy. ANZJ Surg. 2001;71:647-649.
- Lorea P, Baeten Y, Chahindi N, Franck D, Moermans JP. A
severe complication of muscle transfer: clostridial
myonecrosis. Ann Chir Plast Esthet. 2004;49:32-35.
- Voros D. Anaerobic infections of the soft tissues and
bones. Anaerobe. 1997;3:117-119.
- Dunham CM, Coates S. Clostridial septic shock following an
isolated, hepatic gunshot wound. Injury. 1996;4:291-293.
- Stevens DL, Bryant AE. The role of clostridial toxins in
the pathogenesis of gas gangrene. CID. 2002; 35 (Suppl.
1):593-600.
-
Awad MM, Ellemor DM, Boyd RL, et al.
Synergistic Effects of Alpha-Toxin and Perfringolysin O in
Clostridium perfringens-Mediated Gas Gangrene. Infect
Immun. 2001; 12:7904–7910.
- Augustynowicz E, Gzyl A, Ślusarczyk J. Molecular
epidemiology survey of toxinogenic Clostridium perfringens
strain types by multiplex PCR. Scand J Infect Dis. 2000;32
:637-641.
- Strauss MB, Bryant B. Hyperbaric oxygen. Orthopedics.
2002;3:303-310.
|




