|
Abstract
Introduction: Current treatment of
segmental bone defects includes amputation, autograft, bone
transport, free vascularized fibula, and acute shortening. All
have recognized significant complications and morbidity.
Recombinant human bone morphogenetic proteins have been used
successfully in lumbar fusion and acute open tibia fractures.
The purpose of this study was to evaluate the union potential of
recombinant human bone morphogenetic protein-2 (rhBMP-2)
implanted on an absorbable collagen sponge (ACS) in human
segmental bone defects.
Methods: We performed a retrospective analysis using
rhBMP-2/ACS with bone graft substitutes (calcium sulfate or
calcium phosphate) in treating 19 segmental bone defects in 18
patients. Etiology included acute trauma, post-trauma infection,
or nonunion. There were 11 males and 7 females. There were 9
femur fractures, 6 tibial fractures, 2 clavicle fractures, 1
humerus fracture, and 1 ulnar fracture. Ten defects were 100%
circumferential, while 9 were partial defects. Defect length
averaged 4.75 cm, ranging from 1.5 to 8.0 cm. Open fractures
occurred in 14 patients. Failure was determined as need for
further surgical intervention or nonunion. A fracture was noted
as healed by clinical use without pain and radiographic
consolidation.
Results: Bony union occurred in 16 of 19 bone defects,
with a union rate of 84%. Average time to union was 8.4 months
(range 3.5 to 13.5 months). Failure was noted in 3 patients. Two
of these patients were treated early on in the study with
calcium sulfate in association with rhBMP-2/ACS and had
premature resorption of the graft. The third failed patient had
fixation failure at 6 weeks due to non-compliance. No infections
were reported. No clinical reactions from the rhBMP-2 were
reported.
Discussion and Conclusion: rhBMP-2 has the capability to
heal critically sized bone defects in a variety of patients,
with a success rate of 84% in our study. This can be done
without the morbidity associated with auto-graft or many of the
complications of other treatment. Treatment can also occur in a
timely fashion given the severity of injury in some cases.
J.Orthopaedics 2006;3(2)e2
Introduction:
Management of segmental bone defects
resulting from trauma can present the surgeon with a tremendous
challenge. The impact on the patient is often significant as
well, resulting in either limited function of the extremity or
major risk and morbidity to many of those treated. The
recommended treatment for defects less than 5 cm in length is
rigid fixation and autogenous bone 1,2,3,4. The disadvantages
of using autograft bone include the blood loss associated with
harvest and morbidity at the donor site 5,6,7,8. For bony
defects greater than 5 cm’s, historical options have included
amputation, bone transport, or vascularized fibular transfers
9,10,11,12.
All of these options have cited potentially significant
morbidity associated with treatment. Donor site morbidity of
fibular graft site has been reported in 19% of cases 6 and
fracture through the graft can occur in 25% of patients 7.
Recent literature suggests that recombinant
human bone morphogenetic proteins (rhBMP) may be a viable
alternative to autograft bone. With significant osteo-inductive
effects 13,14, rhBMP-2 soaked onto an absorbable collagen sponge
(rhBMP-2/ACS, INFUSE® Bone Graft, Medtronic Sofamor Danek,
Memphis, TN) has already shown to be effective in spinal fusion
15. BMP has been shown to promoted bone formation in critical
sized bone defects in several animal models 16,17,18,19, and has
been used successfully as part of composite grafting for
craniofacial reconstruction 20. Johnson et al reported on the
use of human BMP extracts combined with autograft to treat six
segmental defect patients; all patients developed solid union,
with an average time to union of 4.7 months 21. Recently,
rhBMP-2/ACS has also been approved by the FDA as an adjunct for
the treatment of open tibia fractures 22. The purpose of this
study was to retrospectively review the union rate of critically
sized segmental long bone defects using rhBMP-2 in combination
with a bone substitute.
Material and Methods :
At our institution, we retrospectively
reviewed the charts and radiographs of 19 patients (20 defects)
who had been treated with recombinant human bone morphogenetic
protein-2 on an absorbable collagen sponge along with either
calcium phosphate granules (MastergraftÒ, Medtronic) or calcium
sulfate pellets (OsteosetÒ Wright Medical) in the treatment of
segmental long bone defects. The calcium phosphate granules
were made of a composite of 15% hydroxyapatite (HA) and 85%
beta-tricalcium phosphate (β-TCP). One patient died of unrelated
causes and was not used in the results, leaving 19 defects in 18
patients. Patient ages ranged from 16 to 71 with a mean of
41. There were 11 males and 7 females. Major comorbidities
included rheumatoid arthritis, chronic steroid use, diabetes
mellitus, obesity, alcoholism, COPD, and tobacco use (Table 1).
Table 1. Patient Information
|
Age |
Site of Defect |
Size (cm) |
Circumerence (%) |
Open Fracture+ |
Time to Healing |
Comorbid Conditions |
|
28 |
CLAVICLE |
2 |
100 |
NO |
FAILED |
Tobacco, NSAIDS |
|
38 |
TIBIA |
4 |
50 |
III-B |
9 |
Upper Extremity Fracture |
|
55 |
SUPRACONDYLAR FEMUR |
5 |
50 |
II |
8 |
Diabetes Mellitus Type I |
|
58 |
TIBIA |
5 |
100 |
III-B |
FAILED |
Ipsilateral Femur Fracture |
|
58 |
SUPRACONDYLAR FEMUR |
8 |
50 |
III-A |
10 |
Ipsilateral Tibia Fracture |
|
60 |
TIBIA |
5 |
100 |
III-B |
8.5 |
Fibula, Contralateral Femur |
|
34 |
FEMUR |
5 |
100 |
III-A |
FAILED |
Tobacco |
|
79 |
SUPRACONDYLAR FEMUR |
7 |
100 |
I |
DECEASED |
COPD |
|
17 |
ULNA |
8 |
100 |
II |
6.5 |
Ipsilateral Radius Fracture |
|
15 |
TIBIA |
1.5 |
100 |
II |
10 |
None |
|
71 |
TIBIA |
5 |
50 |
I |
3.5 |
Tobacco, COPD, Diabetes Mellitus Type II, MI x 3 |
|
59 |
TIBIA |
5 |
100 |
III-B |
9 |
Healed ipsilateral femur fracture |
|
35 |
CLAVICLE |
4 |
100 |
NO |
12.5 |
None |
|
48 |
TIBIA |
5 |
100 |
III-C |
10 |
None |
|
22 |
SUPRACONDYLAR HUMERUS |
4 |
75 |
III |
13.5 |
None |
|
52 |
SUPRACONDYLAR FEMUR |
2.5 |
100 |
NO |
8 |
Hypothyroidism |
|
38 |
SUPRACONDYLAR FEMUR |
4 |
50 |
III-A |
6 |
Tobacco, NSAIDS, Ipsilateral Quadriceps Tear |
|
46 |
FEMUR |
7 |
100 |
NO |
9 |
Chronic Steroids, Asthma |
|
40 |
TIBIAL PLAFOND |
3 |
50 |
III-B |
9 |
Tobacco |
|
23 |
FEMUR |
4 |
50 |
II |
6 |
None
|
* Same patient with multiple defects
+ Gustillo-Anderson Classification
α Defect 4 Re-grafted
The defects were caused by trauma in 16, were
post-infectious in 2, and one nonunion. The defects were all in
long bones and included 9 femurs, 6 tibias, 2 clavicles, 1
humerus, and 1 ulna. Defect size was measured intra-operatively
with or without contra-lateral comparison radiographs ranged
from 1.5 to 8.0 cm, with a mean of 4.75 cm. The cortical
defects were 100% circumferential in 10 patients, while 9
patients involved loss between 50-99 %. At initial trauma 14 of
the defects had been open fractures.
All wounds that were open or were infected
were treated with multiple irrigations and debridements,
intravenous antibiotics as appropriate, and soft tissue
coverage. Delayed grafting then took place at a standard of 6
weeks if the wounds were clinically free of infection. The
procedures involved early internal fixation either with plates
and screws or intramedullary rodding techniques as necessary.
The technique of grafting involved rhBMP-2
soaked into an absorbable collagen sponge (used as a carrier for
the BMP) for 20 minutes. The next step was to then place the
bone substitute on top of the sponge and roll it into what we
called a ‘burrito’. This was then placed into the defect. Size
was determined based on need. Calcium phosphate was used in 16
defects, and calcium sulfate was used in 3. The wounds were
then closed in standard fashion. Post-operative care involved
cast immobilization and non-weight bearing where applicable.
Union was determined by consolidation of the
graft and formation of interlacing trabeculae. Failure was
determined by reabsorption of graft material, lack of
progression, or fixation failure. The patient charts were also
evaluated as to clinical function of the extremity.
Results :
Radiographic evidence of union, which
consisted of trabecular formation and consolidation of the
fracture, occurred in 16 of 19 defects (84%). Plain radiographs
taken at routine intervals show maintenance of graft and
progressive consolidation in the successful grafts, as shown in
the example in Figure 1. There were 3 failures, which included
resorption of the graft in 2 cases, and fixation failure in one
patient.
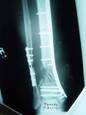 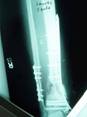 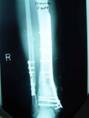
1a. 1b. 1c.
Figure 1 a-c. a) Radiographs of post-traumatic segmental
defect after wash out, internal fixation, and placement of
antibiotic beads. b) Radiographs 3 months after grafting
technique showing interval consolidation. c) Consolidation of
defect at 10 months.
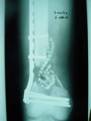 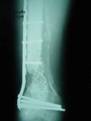 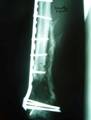
2a. 2b. 2c.
Figure 2 a-c. a) Initial injury post internal fixation
and antibiotic bead placement. b) Same injury 2 months post
grafting technique. c) Same injury 5 months post-grafting with
reabsorbtion of the calcium sulfate graft material.
The average time to clinical union, which
included radiographic union and clinical functional use of the
extremity, was 8.4 months. Union times ranged from 3.5 months
up to 13.5 months. There was no correlation between time to
union and the defect size. There was no correlation between
those who abused tobacco and time to union. None of the other
comorbidities (Table 1) correlated with time to union.
Failure of the graft to unite occurred in 3
defects. In the first case, the patient was treated for a
grade III-B open tibia fracture and ipsilateral grade III-A
supracondylar femur fracture. He was treated with multiple I +
D’s, intramedullary rodding and gracillis rotation flap for
coverage for the tibia and internal fixation for the femur. He
then underwent delayed grafting with rhBMP-2/ACS and calcium
sulfate at approximately 11 weeks post-injury for both defects.
At 5 months post-grafting, the tibial graft had reabsorbed and
the gap remained. He underwent repeat grafting (the second time
with calcium phosphate and rhBMP-2/ACS) and healed at 9 months.
The second failure, shown in Figure 2,
occurred in a 34 yo male with a grade III-A open supracondylar/intercondylar
femur fracture. He had early internal fixation along with
multiple debridements and antibiotic beads. At 7 weeks
post-injury, he underwent grafting with rh-BMP-2/ACS along with
calcium sulfate. At 5 months post-injury, the graft had
reabsorbed.
The 3rd failure involved a 28 yo female with
a symptomatic clavicular non-union. Approximately 6 weeks after
grafting with BMP and tri-calcium phosphate, the internal
fixation failed due to patient non-compliance.
Discussion :
As discussed, the difficulty of treating
post-traumatic and post-infectious segmental bone defects
continues to be a troublesome problem. Clinicians continue to
search for new ways of treatment given the frequent
complications that ensue with current treatment regiments. BMP
bone grafting has the potential to be a valuable new option for
segmental defects, and has already been shown useful in the
treatment of spinal fusion, nonunion of difficult fractures, as
well as in the treatment of open tibial fractures 15,22,23.
Bone morphogenetic protein has been studied
extensively in animals involving segmental defects. Bostrom et
al used a 2-cm ulnar defect model in rabbits to show dose
dependent bone formation, showing union in all defects that were
given the highest dosage 16. Moreover, histological analysis
demonstrated normal bone formation when using rhBMP-2/ACS.
Yasko et al also found dose related healing in a rat model 18.
Cook et al demonstrated 89% (25/28) union rate at 12 weeks in
2.5-cm canine ulnar segmental defects using rh-BMP-7 (OP-1).
Within the same time frame, the torsional strength of the new
bone was rated at 65% of intact ulnas 17. Sciadini and Johnson
found healing comparable to auto graft when rhBMP-2/ACS was used
in the same canine defect model 19.
Use of rhBMP in humans has to date involved
spinal fusion, healing of tibial nonunion, and use in open
tibial fractures. A prospective, randomized trial involving
patients with lumbar degenerative disc disease by Burkus et al
compared the use of rhBMP-2/ACS vs iliac crest autograft in
lumbar fusion, along with the use of lumbar cages. They found a
higher fusion rate (94.5 vs 88.7%) at 24 months in the group
treated with BMP, along with shorter operative times, less blood
loss, and similar clinical outcomes 15. The control group had 8
adverse events related to the iliac crest bone donor site. In a
more recent trial, Govender et al evaluated the use and safety
of rhBMP-2/ACS in a randomized, single blinded study involving
open tibia fractures. The patients were stratified according to
severity, and either the standard procedure (involving local
wound care with irrigation and debridement, and a static locked,
reamed, IM nail) or the standard procedure along with the use of
BMP at two different concentrations soaked on to the ACS carrier
(1.5 mg/mL vs 0.75 mg/mL). Results demonstrated a 44%
reduction of the risk of secondary intervention in the group
treated with 1.5 mg/mL rhBMP-2/ACS when compared with controls.
Healing rates of 58% (BMP) vs 38% (standard treatment) were
also observed at 12 months 22.
Recently, Jones et al presented results from
a small clinical trial involving rhBMP-2/ACS used as part of a
staged bone grafting procedure.24 That study involved the
treatment of 30 tibial fractures with traumatic bone loss of 1
to 5 cm in length. Patients were randomized to receive iliac
crest auto graft or allograft plus rhBMP-2/ACS. The author
concluded that rhBMP-2/ACS combined with allograft yielded
similar healing to autogenous bone graft.
In this study 2 out of the 3 patients treated
with calcium sulfate and rhBMP-2/ACS experienced resorption of
the graft material, resulting in failure. It is theorized that
the increased cellular activity induced by the rhBMP-2 led to
the resorption. The effect was not observed with the slower
resorbing calcium phosphate granules. Surgeons should be
cautious of using any fast resorbing material, such as a calcium
sulfate, in the presence of a BMP product. If the 3 calcium
sulfate treated patients are removed from the analysis, then
bony union occurred in 15 out of 16 defects (94%) treated with
calcium phosphate and rhBMP-2/ACS.
We report here a retrospective review
utilizing rhBMP-2/ACS combined with a bone substitute in
segmental long bone defects with reasonable clinical results.
The rhBMP-2/ACS implant has been shown to be safe in humans and
has excellent osteo-inductive effects. We will continue to
evaluate patients critically for the possible use of this
technique.
Conclusion:
In summary, Metal-on-metal resurfaced hips
with appropriate case selection can yield satisfactory results
in the young and active patients with abnormal coxanatomy. This
technique used in three patients with successful outcome and
averted the need of structural graft augmentation.
Reference :
-
Dabezies EJ, Stewart WE, Goodman FG, Deffer
PA. Management of segmental defects of the radius and ulna. J
Trauma. 1971; 11:778-88.
-
Grace TG, Eversmann WW Jr. The management
of segmental bone loss associated with forearm fractures. J
Bone Joint Surg Am. 1980;62:1150-5.
-
Moroni A, Rollo G, Guzzardella M, Zinghi G.
Surgical treatment of isolated forearm non-union with
segmental bone loss. Injury. 1997;28:497-504.
-
Ring D, Allende C,
Jafarnia K, Allende BT, Jupiter JB. Ununited Diaphyseal
Forearm Fractures with Segmental Defects: Plate Fixation and
Autogenous Cancellous Bone-Grafting. J Bone Joint Surg Am.
2004; 86:2440-2445.
-
Younger EM, Chapman MW.
Morbidity at bone graft donor sites. J Orthop Trauma.
1989;3:192-5.
-
St John TA, Vaccaro AR,
Sah AP, Schaefer M, Berta SC, Albert T, Hilibrand A. Physical
and monetary costs associated with autogenous bone graft
harvesting. Am J Orthop. 2003;32(1):18-23.
-
Ahlmann E, Patzakis M,
Roidis N, Shepherd L, Holtom P. Comparison of anterior and
posterior iliac crest bone grafts in terms of harvest-site
morbidity and functional outcomes. J Bone Joint Surg Am.
2002;84-A(5):716-20.
-
Vail TP, Urbaniak JR.
Donor-site morbidity with use of vascularized autogenous
fibular grafts. J Bone Joint Surg Am. 1996;78:204-211.
-
Jupiter JB, Bour CJ, May
JW. The Reconstruction of Defects in the Femoral Shaft with
Vascularized Transfers of Fibular Bone. J Bone Joint Surg Am.
1987; 69: 365-374.
-
Heitann C, Erdmann D,
Levin LS. Treatment of Segmental Defects of the Humerus with
an Osteoseptocutaneous Fibular Transplant. J Bone Joint Surg
Am 2002; 84: 2216-2223.
-
Song HR, Kale A, Park HB,
et al. Comparison of Internal Bone Transport and vascularized
Fibular Grafting for Femoral Bone Defects. J Orthop Trauma.
2003; 17(3): 203-211.
-
Bowers KW, Edmonds JL,
Girod DA, Jayaraman G, Chua CP, Toby EB. Osteocutaneous Radial
Forearm Free Flaps: The Necessity of Internal Fixation of the
Donor Site Defect to Prevent Pathologic Fracture. J Bone Joint
Surg Am 2000; 82: 694.
-
Zagula H D, Buck DC,
Brekke J, Wozney J M, Hollinger JO. Bone formation with the
use of rh BMP-2 (Recombinant Human Bone Morphogenic Protein).
J Bone Joint Surg Am 1997; 79A (12): 1778-1790.
-
Chang H, Jiang W,
Phillips FM, et al. Osteogenic Activity of the Fourteen types
of Human Bone Morpogenic Proteins (BMP’s). J Bone Joint Surg
Am 2003; 85: 1544-1552.
-
Burkus JK, Gornet MF,
Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion
using rhBMP-2 with tapered interbody cages. J Spinal Disord
Tech. 2002; 15:337-49.
-
Bostrum M, Lane JM, Tumin
E, et al. Use of Bone Morphogenic Protein-2 in the Rabbit
ulnar Nonunion Model. Clin Orth. 1996; 1(327): 272-282.
-
Cook SD, Salkeld SL,
Brinker MR, Wolfe MW, Rueger DC. Use of an Osteoinductive
Biomaterial (rhOP-1) in Healing Large Segmental Bone Defects.
J Orthop Trauma. 1998; 12(6): 407-417.
-
Yasko AW, Lane JM,
Fellinger EJ, Rosen V, Wozney JM, Wang EA. The Healing of
Segmental Bone Defects induced by Recombinant Human Bone
Morphogenic Protein (rh-BMP-2). A Radiographic, Histological,
and Biochemical Study in Rats. J Bone Joint Surg Am 1992;
74(5): 659-670.
-
Sciadini MF, Johnson KD.
Evaluation of recombinant human bone morphogenetic protein-2
as a bone-graft substitute in a canine segmental defect model.
J Orthop Res. 2000;18:289-302.
-
Desilets CP, Merden CJ,
Patterson AL, et al. Development of Synthetic Bone-Repair
Materials for Craniofacial Reconstruction. J Craniofac Surg.
1990; 1: 150-153.
-
Johnson EE, Urist MR,
Finerman GA. Repair of segmental defects of the tibia with
cancellous bone grafts augmented with human bone morphogenetic
protein. A preliminary report. Clin Orthop Relat Res. 1988
-
Govender S, Csimma C,
Genant H K, et al. Recombinant Human Bone Morphogenetic
Protein-2 for Treatment of Open Tibial Fractures: A
Prospective, Controlled, Randomized Study of Four Hundred and
Fifty Patients. J Bone Joint Surg Am 2002; 84: 2123 - 2134.
-
Friedlaender BE, Perry
CR, Col JD, et al. Osteogenic Protein-1 (Bone Morphogenic
Protein-7) in the Treatment of Tibial Nonunions. J Bone Joint
Surg Am 2001; 83-A Supp 1: 151-158.
-
Jones AL, Bucholz RW,
Bosse MJ, et al. Prospective, randomized comparison of
rhBMP-2/ACS in combination with allograft versus autogenous
bone graft in healing diaphyseal tibial fractures with
traumatic bone loss. Trans Orthopaedic Trauma Association.
Poster #17, 2004.
|








