|
ABSTRACT
Bone morphogenic proteins
(BMP) have shown significant potential in enhancing spinal
fusions and bone formation at non-union sites. Animal studies
and limited human studies have proven their efficacy as an
alternative or enhancer of autologus bone graft in bone
regeneration. Optimal dose and carrier especially in complex
scenarios like recalcitrant non unions posterolateral spinal
fusion still remain an important issue where the use of BMP has
not been entirely successful. Their use in fields like articular
regeneration, joint replacement and chronic renal failure is
also being aggressively investigated. This review article
intends to give brief information on the biology and basic
science behind BMPs and provide an update on the current
research data on various clinical applications of BMPs.
Key
Words: Bone morphogenic protein, Spine fusion,
Fracture healing, Bone regeneration, rh BMP 2, OP –1
J.Orthopaedics 2005;2(4)e3
Introduction
Use of biofactors for bone
regeneration has revolutionized the management of fracture and
spinal fusion. Various biological factors, such as bone
morphogenic proteins (BMP), fibroblast growth factors (FGF),
platelet-derived growth factor (PDGF), insulin like growth
factors (IGFs) and LIM mineralization protein-1, have been
investigated for application in bone regeneration and skeletal
repair. Despite remaining the gold standard for most orthopaedic
procedures, autologus bone graft suffers from significant
disadvantages (Table 1). Different approaches are being tried to
achieve sound bone regeneration (Table 2) and the look out for
idea bone graft continues (Table 3).
Table 1:Disadvantages of autogenous bone
graft
|
Limited availability. |
|
Postoperative pain at the operative
site |
|
Potential injury to the lateral femoral
cutaneous nerve. |
|
Potential injury to superior gluteal
artery. |
|
Postoperative hematoma. |
|
Potential for infection at the
operative site |
|
Possibility of the gait disturbance. |
Table 2: Current approach towards Bone
regeneration:
Osteogenic Methods:
Autogenous Bone grafts.
Allogenic bone grafting.
Autogenous bone marrow grafting.
Osteoinductive Method:
Bone Morphogenic proteins.
Platelet rich plasma containing growth factors like
PDGF, IGF I and II, TGF beta.
Osteoconductive methods:
Calcium based ceramic grafts.
Calcium based collagen substitutes.
Synthetic polymers.
Bioactive ceramics and glasess.
Systemic agents:
Prostaglandins.
Osteogenic proteins present in systemic circulation.
Bone Morphogenic proteins
or BMP as a viable substitute to autologus bone graft has been
subject of intense research in last few decades and has followed
a long and iterative process to provide a burden of proof for
clinical use at present time (Fig 1). They are by far the most
extensively studied orthobiologic product in history and over
2000 peer-reviewed publications in worldwide literature have
studied their application. The long awaited approvals for the
clinical use and commercial availability have only recently been
granted. The research in field of BMP is being pursued
relentlessly and studies on their mechanism of action, optimal
formulations, and alternative uses continue. This review article
intends to give brief information on the biology and basic
science behind BMPs and provides a latest update on the research
data on various applications of BMPs for clinical use.
Figure 1: Burden of proof assessment for
rh BMP 2
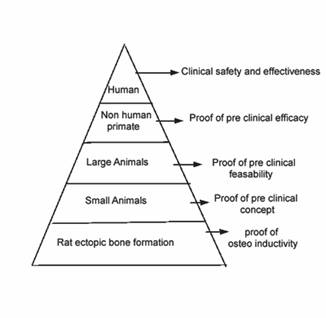
Table 3: Features of an ideal bone graft
substitute
|
Have results as good as or better than
autograft in achieving union. |
|
Be cost effective |
|
Have no immunogenicity |
|
Have handling characterstic familiar to
surgeon |
|
Resorb with a predictable degradation
time. |
|
Act locally without any or negligible
systemic side effects |
|
Be osteoconductive and osteoinductive
with a potential of supplying or attracting osteogenic cells |
|
Not interfere with modern imaging
modalities |
|
Produces non exothermic reaction when
implanted so as to prevent heat damage to antibiotics and
growth factors. |
History
Dr Marshall Urist in 1965
pioneered the concept of presence of a substance that is
naturally present in the bone and is responsible for
regeneration and repair activity in the bone, he called this
substance bone morphogenic protein (BMP), later also known as
osteogenic protein or OP. Since then the path breaking research
provided newer insights into the nature of bone biology and the
break through in the recombinant technology made commercial
availability of BMP products a reality (Table 4).
Table 4:History of evolution of Bone
Morphogenic protein:
|
1965 |
Marshall Urist’s discovery that
demineralized bone matrix (DBM) can induce bone formation. |
|
1971 |
Urist develops concept of Bone
Morphogenic Proteins (BMP’s) |
|
1972 |
Hari Reddi, Huggins find bone induction
is a sequential cascade with multiple steps. |
|
1981 |
Reddi and Kuber Sampath do associated
extraction and reconstitution of BMP activity for bioassay.
|
|
1991 |
Orthopedic surgeons use BMP,s for the
first time as Demineralized Bone matrix (DBM) is available
for commercial use. |
|
October 2001 |
FDA grants humanitarian device
exemption (HDE) approval for Osteogenic protein –1 (OP-1; rh
BMP –7) |
|
November 2002 |
FDA approves rh BMP 2 (INFUSE) for a
single level spine fusion. |
|
April 2004 |
FDA grants HDE approval for OP-1 putty
for revision spinal fusion. |
|
May 2004 |
FDA approves rh BMP-2 (INFUSE) for
treating acute open tibial shaft fractures. |
These proteins have been
isolated from the bones of a variety of mammals - mouse, rats,
bovine, monkey and man and also from clonal osteogenic sarcoma
lines. In 1979, Urist et al showed that BMP can be extracted
from animal cortical bones by digesting the demineralized bone
matrix with bacterial collagenase and solubilization of the
digest in a neutral ethylene glycol and a salt mixture. The
extracted BMP was found to induce bone formation in not only the
same species but also in other species. The human BMP was later
extracted by Bauer and Urist using a 4M guanidine hydrochloride
solution, this extracted substance was shown to induce bone
formation in thigh muscles of athymic nude mice.
In 1980’s bone inductive
preparations were purified from bovine bone in sufficient
quantity and purity to provide amino acid sequence data. Using
these sequences, nucleic acid probes were generated and used for
the identification and characterization of DNA sequence encoding
these proteins. With advent of better isolation techniques and
the research leading to recombinant cloning techniques, a large
number of molecules that form part of the BMP family have been
described and have provided a vital impetus to research in this
field (Table 5).
Table 5: BMP and their alternative
names:
|
BMP Number |
Other Names |
|
BMP-2 |
BMP 2A |
|
BMP- 3 |
Osteogenin |
|
BMP- 3B |
GDF 2B |
|
BMP-4 |
BMP 2B |
|
BMP-5 |
- |
|
BMP-6 |
VGR 1 |
|
BMP-7 |
OP1 |
|
BMP-8 |
OP2 |
|
BMP-8B |
OP3 |
|
BMP-9 |
- |
|
BMP-10 |
- |
|
BMP-11 |
GDF 11 |
|
BMP-12 |
GDF 7, CDMP 3 |
|
BMP-13 |
GDF 6, CDMP 2 |
|
BMP-14 |
GDF 5 CDMP1 MP52 |
|
BMP-15 |
- |
|
BMP- 16 |
|
The availability of
recombinant human BMP (rh BMP) created an opportunity to assess
the material properties devoid of impurities and without the
potential risk of xenograft reaction during human use. All
except BMP 3 have shown to be osteoinductive. BMP 3 has in fact
shown to be an inhibitor of osteoinductive activity in the rat
assay, this is interesting given the fact that the BMP 3 is the
most abundant BMP in bone.
Bone Morphogenic Protein Classification,
Character and Properties
BMPs are members of the TGF
– beta super family. The super family compromises of proteins
that are coded for by a 45-gene sequence that has a highly
characteristic conserved 7 Cysteine motifs in their mature
domain. This super family of proteins contains: five isoforms
of TGF –Beta (TGF beta 1 through TGF beta 5), the BMPs, growth
differentiation factors (GDFs), activins, inhibins and Mullerian
inhibiting substance. The superfamily has impact on a wide array
of cellular activities including growth, differentiation and
extracellular matrix formation.
BMP is the largest sub
group belonging to the TGF beta superfamily. They are
synthesized and stored as large dimeric proteins in the
cytoplasm and cleaved by proteases during secretion. The
structure of BMPs that has been most extensively studied in OP 1
is one of a polypeptide containing 431 amino acids. The crystal
structure described for OP1 and BMP 2 consists of a “hand shaped
structure” comprising two fingers of anti parallel beta strand
and an alpha helical region at the heel of the palm.
Signaling pathway:
BMP exert their effect through activation of transmembrane
heteromeric receptor complex formed by types I and type II
serine /threonine kinase polypeptides, also known as the BMP
receptor (BMPR) type I A and I B and BMPR Type II. The
activated receptor kinases in turn phosphorylate the
transcription factors Smad 1, 5, and 8. The phosphorylated Smads
then forms a heterodimeric complex with Smad 4 in the nucleus
and activate the expression of target genes in concert with co
activators.
BMP localization:
Traditionally BMP were considered localized to bone but
subsequent studies have shown that BMPs are expressed in most
other tissues and throughout the embryonic development. Some of
these members of BMP family have also been mapped to different
chromosomes loci’s: BMP 2 (Chromosome 20), BMP 3 (Chromosome 4),
BMP 4 (Chromosome 14), BMP 6 (Chromosome 6), BMP 7 (Chromosome
20), BMP 8 (Chromosome 1), BMP 15 (chromosome X).
Biological Activity:
BMP are pleiotropic regulators orchestrating various sequential
cellular response: chemo taxis of cells, mitosis and
proliferation of progenitor cells, differentiation into
chondroblasts, cartilage calcification, vascular invasion, bone
formation, remodeling and bone marrow differentiation. BMP also
stimulates extra cellular matrix formation and besides its
osteogenic potential the BMPs have also shown to have an effect
of the development of other organ and tissues particularly those
form through the mesenchymal-epithelial interactions.
Implantation of purified
recombinant BMP with bone collagen matrix in subcutaneous sites
in rats has shown to induce a sequence of cellular event leading
to formation of new bone with all its elements (Table 6). The
BMP stimulates the stem cells to proliferate and differentiate
into chondrocytes. This transformation takes 5 to 7 days,
following which the capillary invasion takes place. The
chondrocytes subsequently hypertrophies and becomes calcified,
and the osteoblasts appear at the implant site. The new bone
formation is seen at 9-12 days and subsequent remodeling and
formation of ossicles and bone formation takes place in next 14
–21 days. This process is identical to the physiologically
occurring enchondral ossification. A process similar to
intramebranous ossification in which stem cells directly
differentiate into the osteoblasts has also been seen with BMP
in some in vitro studies. However this effect may be seen only
at a higher concentration of BMP.
Table 6: Biological action of BMP:
|
Chemotaxis |
Mesenchymal stem cells and other bone
forming cells migrate to the site of implantation. |
|
Proliferation |
Mesenchymal and other bone forming
cells divide and increase in number. |
|
Morphogenesis |
Cells begin to take on the form and
structure of bone. |
|
Neo- angiogenesis |
New blood vessels are formed in the
immature callus. |
|
Calcification |
Osteoblasts produce new mineralized
tissue under biologic influences like mechanical loading and
growth factors. |
|
Maturation |
Some osteoblasts transform into the
osteocytes, the body continues to remodel under local
environmental and mechanical forces, leading to formation of
a normal trabecular bone pattern. |
Development and
production: The extracted BMP from bone was not a
commercially viable option and this prevented the exploitation
of BMP technologies in 80’s and 90’s, but with the evolution of
recombinant technology the commercial development of BMP took
the center stage of the mainstream research in orthopedics. The
recombinant methodology results in extreme pure solutions of a
single BMP. The recombinant technology used to develop and
manufacture BMP involves two steps:
The Specific genes
responsible for carrying code for making BMP in humans were
identified at Genetics institute. Once this gene was identified
and isolated, it was spliced and recombined into the DNA of a
commonly used production cell. This insertion or ‘recombination’
of gene results in formation of a “recombinant”. The recombinant
cell grows and multiplies by a process known as ‘cloning’. This
results in development of a homogenous population of cells
producing a recombinant human bone Morphogenic protein. Batches
of recombinant cells are preserved in several small vials for
future production also known as cell banking. The cell bank is
maintained at –135 degree centigrade for future production of
BMP. The recombinant cells when cultured in optimal media
produce the BMP that after appropriate purification process is
available for commercial use.
The commercially available
BMPs approved currently by US FDA are: rh BMP 2- Infuse (Medtronics
Sofamor Danek, Memphis, Tenesse) and OP1 (Stryker Biotech,
Hopkinton, MA). Other BMP products that are being currently
evaluated for commercial use include BMP –X (Sulzer Biologics,
Wheat Ridge, Colarado), BMP –9 and combinations of animal and
human BMP implant.
Delivery Material:
Carrier for BMP needs to perform a three-fold function:
-
Maintaining a critical
threshold concentration of BMP at implantation site for the
required period (Temporal Distribution)
-
Act as scaffold over which bone growth can occur.
-
Contain the BMP at the localized site and prevent extraneous
bone formation. (Spatial containment).
The delivery material in
addition to these, should be biocompatible and biodegradable,
and allow a rapid neo angiogenesis and invasion by mesenchymal
cells. It should resorb over time as the new bone forms and
remodels. Application specific carrier are being tested that
will enable release of BMP over an adequate period in adequate
concentration. Various materials have been evaluated for local
delivery of BMPs for new bone formation (Table 7). An absorbable
collagen sponge (ACS), reconstituted from bovine tendon, and a
collagen based matrix, derived from demineralized/guanidine –
extracted bovine bone, are two most common delivery materials
currently being used for rh BMP-2 and rh BMP-7 respectively. The
collagen in these delivery materials is a natural component of
the bone and preclinical studies have indicated that they may
also play a role in pharmacological stimulation of local bone by
both proteins. Several recent studies have led to enthusiasm
about injectable solution in a buffered media as an alternative
to solid phase matrix carriers.
Table 7: Various carrier materials
available for BMP:
|
Natural Polymers: Different collagens,
fibrins, Fibronectin, Hyaluronic acids, glycosaminoglycan. |
|
Demineralized Bone matrix. |
|
Synthetic polymers: Poly lactates and
poly glycolic acid (PGA) |
|
Hyaluronic acid gel |
|
Ceramics: Hydroxyappatite, Tricalcium
phosphates. |
|
Allograft. |
|
Non ceramic Inorganic material: Calcium
phosphate based cements (CPCs), Calcium sulfates, metals and
bioglass. |
|
Newer delivery models being
investigated: Depot injectable carriers, viral vectors, gene
guns, oral small molecule targets, conjugated osteogenic
factors. |
Dosage Related Toxicity:
There have been many preclinical toxicity studies to evaluated
acute and systemic toxicity, bio distribution, reproductive
toxicity and carcinogenity of BMPs. BMP has demonstrated
excellent safety profile in most studies. Studies have even used
up to 1000 times the dose that are used clinically and did not
find any adverse drug reaction. The preclinical toxicity study
have shown that direct injection of high doses of rh BMP 2
(5.3mg/kg) into the blood stream did not have significant
adverse effects. The possible reason behind the lack of adverse
drug effect with high doses of BMP may be its extremely short
half-life that has been found to be less than 1 min. After
orthotopic injection the maximum blood level that rh BMP 2
achieves is only 0.1% of the implanted amount, this amount also
disappears very rapidly from the circulation. Another study that
investigated the adverse effect of BMP after direct application
to nervous tissue, found no adverse physiologic or permanent
histologic effects on these tissues. This is encouraging in view
of the fact that certain application of BMP in spine surgery may
involve such a scenario.
Carcinogenicity:
There is no evidence that the BMP is carcinogenic. Conversely,
it has shown anti proliferative effect in vitro on human breast,
ovary, lung and prostate cells. Pre clinical safety studies have
shown to have inhibitory effect on the human osteosarcoma,
prostate, lung, breast and tongue carcinoma line.
Antibodies formation
against BMP: Studies have documented presence of antibodies
to rh BMP 2 in patients treated with rh bmp 2 collagen sponge in
tapered cages for anterior spinal fusions to extent of 0.7%,
raising the possibility that its use may not be effective in all
group of patients. However this is still not an issue yet with
its use in clinical practice.
Ectopic Bone Formation:
The formation of ectopic bone outside the desired field is also
a potential concern that is aggressively being investigated.
Paramore et al evaluated the toxicity of OP-1 by placing OP-1
into the epidural space after laminectomy and posterolateral
fusion in a dog model. They found that animals with OP –1
implantation demonstrated bone formation adjacent to spinal cord
that caused mild spinal cord compression. The spinal cord
histology however, showed no evidence of spinal cord
inflammation or neuron cell death. Some other animal studies
however did not find any bony encroachment on the exposed thecal
sac after laminectomy and intertransverse arthrodesis with the
use of rh BMP 2 in non-human primate model.
In a pilot human clinical
study in which cages filled with rh BMP 2 in a collagen carrier
were inserted through a posterior laminectomy approach, several
patients demonstrated formation of heterotopic bone in the
spinal canal posterior to the fixation device and tract of their
insertion. There were no clinical implications resulting from
these ectopic site bone formation but in view of these findings
the study was halted before completion. These findings of
ectopic bone formations was also corroborated in a similar dog
study. The present consensus based on these limited available
information has not suggested limitation of BMP use after repair
of dural tears or open laminectomy defects.
Dose and Concentration:
The dose and concentration of BMP required varies from species
to species (Table 8) and from fusion site to fusion site. In
humans, for anterior interbody fusion a total dose of 4.2 –12 mg
of rh BMP 2 at concentration of 1.5mg/ml is recommended. The
recommended total dose of OP 1 as humanitarian device exemption
for recalcitrant posterolateral fusion non union is 7 mg for
both sides. For inter transverse arthrodesis, the suggested dose
of rh BMP 2as based on pilot clinical trials is 20mg on each
side at a concentration of 2.0mg/ml used on a carrier containing
60% hydroxyapatite and 40% tricalcium phosphate granules. The
recommended dose of OP1 for recalcitrant long bone non-unions is
7mg or two vials (each containing 3.5 mg reconstituted with 1gm
of type I bovine collagen resulting in a net 4 ml volume) for
implantation at the non-union site.
Table 8: Rh BMP 2 Concentration in
different species
|
Species |
Rh BMP 2 Concentration |
Approximate time to form Bone |
|
Lower order Animal |
0.01-0.05 mg/cc |
2-3 weeks. |
|
Canine |
0.75 mg/cc |
6-8 weeks |
|
Non Human primate |
0.75-1.50 mg/cc |
3-6months |
|
Human |
1.50mg/cc |
6-12 months |
Clinical application
The development of BMP
followed a long iterative trial and error process in the
preclinical models. First studied as implants in subcutaneous
sites in rabbit that produced ectopic bones, the early surgical
studies for evaluation of the osteoinductive properties were
done on large critical sized diaphyseal segmental defects in
rats, rabbits, dogs, sheep and non-human primates. These studies
showed that implantation of the BMPs carrier matrices in these
defect led to bone formation that was biomechanically and
biologically sound. BMP was also shown to accelerate bone
formation and repair process in non critical size defects in
closed fracture models that showed an early return of strength
and stiffness. Various clinical scenarios where BMP are
currently approved or are being aggressively being are described
below:
Anterior interbody
Fusion:
Preclinical studies: The first pre clinical interbody cage study
was done by Sandhu et al who compared single level anterior
lumbar interbody fusion rates at 6 months in sheep models using
cylindrical threaded titanium cages filled either with iliac
crest graft or rh BMP 2 on collagen sponge. Out come were
determined using radiographs, biomechanical tests and histologic
analysis. Radiographically all animals achieved fusion, but the
groups differed on the quantity and quality of bone formation
histologically. Compared to 37% histologic fusion rates at 6
months in autograft filled cage group, 100% fusion rates were
seen with cages filled with rh BMP 2 on collagen sponges. In
addition the rh BMP 2 group fusion masses had less fibrous in
growth.
Other animal studies showed
a similar results: Zdeblick et al used a titanium (BAK) cage in
a goat model and demonstrated a fusion rates of 95% when the
cages were filed with rh BMP 2 in contrast to 48% fusion rates
when the cages were filled with autograft.. Boden et al later
performed the study using rh BMP 2 on the collagen sponge in a
titanium lumbar interbody cages at varying dose in rhesus monkey
and it was based on his results that a dose of 1.5mg/ml was
selected for subsequent human trials.
Human studies: A
prospective, multi center, randomized trial in humans was done
to evaluate the efficacy of rh BMP 2 on collagen sponge in a
lumbar tapered cage (INFUSE / LT cage, Medtronic Sofamor Danek,
Memphis, USA) for a single level interbody fusion. A total of
143 patients were enrolled in the study group and 136 patients
were enrolled in the control group for this study. The
experimental group was treated with LT cage with INFUSE and the
control group was treated with same cage filled with iliac crest
auto graft. At six months 99.2% (128/129) patients showed
successful radiographic fusion compared to 96.7% (119/123)
patients in the control autogenous graft. At 2 years all
patients (117/117) treated with INFUSE showed a successful
radiographic fusion compared to 97.2% (99/102) in the control
autogenous group. The clinical improvement that was defined by a
15-point improvement in Oswestry score also followed a similar
trend. While 76.9% of patients in the experimental group had
successful clinical out come, 75.2% had a positive clinical
outcome in the control group.
Another prospective,
multicentered randomized trial using allograft bone dowel
instead of the lumbar tapered cage, studied the efficacy of rh
BMP 2 (INFUSE). A total of twenty-three patients were enrolled
in the control group and twenty-four patients were enrolled in
the experimental group. All patients under went a single level
anterior discectomy and fusion via an open approach. At one
year, 83% of patients treated with INFUSE had more than a 15
point improvement in their Oswestry scores compared with only
58% in the control group. Both group had 90% fusion rates at one
year. The blood loss was significantly less (p=0.026) in the
experimental group than in the control group.
Posterior Lumbar
Interbody Fusion (PLIF):
Preclinical Studies: Magin and Delling performed a posterior
interbody fusion and supplemental transpedicular instrumentation
study in 30 sheep interbody fusion model. They compared OP-1
(3.5 mg of rh OP1 to 1g of bovine collagen), an osteoconductive
HA bone graft substitute and autograft. The sheep fusion was
assessed using CT, radiographs, mechanical testing and
histology. The amount of bone formed using OP-1 was
statistically higher in OP-1 group as compared to autograft and
HA treated group. Mechanical testing and histology also
confirmed that the maturity and stiffness of the fusion in OP-1
treated group was higher than HA group.
Chirossel at al compared
fusion rates with OP1 and autograft in a sheep model using
either a polyethereketone (PEEK) cages or a titanium cage. 22
sheeps were evaluated for fusion using radiographs,
Histomorphometry, flurochrome labeling, CT imaging and
histology. At 24 weeks, solid boney fusion was seen in three of
four titanium cage/autograft animals, three of five-titanium
cage/rh OP 1animals, two of four PEEK/autograft animals and four
of five PEEK/OP-1 animals. These numbers were too small for
statistical significance but histology showed a more mature
trabecular bone within fusion site in the group of animal
treated with OP-1.
Human studies: A
prospective, randomized controlled trial was done to compare the
efficacy of rh BMP 2 (INFUSE) and autologus graft when used with
cylindrical, threaded cages (INTERFIX, Medtronic Sofamor Danek).
These devices were used for single level posterior lumbar
interbody fusion, performed via a posterior laminectomy
approach. Sixty-seven patients were enrolled at 14
investigational centers, thirty-three under went PLIF with
INTERFIX and autogenous bone graft, while thirty-four under went
PLIF with INTER FIX and rh BMP2.
At twenty four months
follow-up period, The fusion rates in the experimental rh BMP 2
group was 92.3% compared to 77.8% in the control autogenous
group. Interestingly both groups had a high number of tobacco
users: 52.9% of the thirty-four in the experimental group and
45.5% in the control group. Another FDA approved study is under
way to study the use of rh BMP 2 with impacted interbody devices
stabilized with posterior instrumentation.
Anterior Cervical
discectomy and Fusion: rh BMP 2 (INFUSE) has also been
studied for application in cervical spine interbody fusion. The
prospective, randomized controlled study enrolled thirty three
patients from four different centers. The study involved the
implantation of machined fibular ring allograft (Cornerstone;
Medtronic Sofamor Danek) filled either with auto graft or rh BMP
2. Eighteen patients in the experimental group received the rh
BMP 2 and fifteen in the control group were treated with
autograft. All patients had fused radiographically at six
months; however the mean blood loss in rh BMP 2 group was less
(91.4 ml) compared to the control group (123.3 ml).
Posterolateral fusions:
In contrast to the interbody spinal fusions, the posterolateral
fusion poses a far more complex challenge. With the structural
containment that the interbody cages provide gone, the pre
clinical studies found that the collagen sponge did not serve an
adequate delivery medium for BMPs in higher primate animals.
Animal studies: Schimandel
et al compared autogenous bone graft and rh BMP 2 in a rabbit
posterolateral fusion model. Inspection, manual palpation,
radiography, histology, and biomechanics testing were used to
assess the fusion. All rabbits implanted with recombinant human
bone morphogenetic protein-2 achieved solid spinal fusion as
confirmed by manual palpation and on radiographs, whereas only
42% of the autograft control fusions were solid. Fusions
achieved with recombinant human bone morphogenetic protein-2
were found to be biomechanically stronger and stiffer than
fusions achieved using autogenous bone graft. Sandhu et al
performed a similar fusion study in a dog posterolateral fusion
model and demonstrated 100% fusion rates with use of rh BMP 2 on
collagen sponge as compared to 0 % fusion rates in the autograft
group. These results were replicated in a subsequent trial where
BMP induced fusion even in absence of decortication.
Cook et al compared the
fusion rates using either OP 1 (2.8 mg of rh OP 1 to 800 mg of
bovine collagen) or auto graft in a dog model. All OP1 treated
levels showed a stable fusion mass by 6 weeks and complete
fusion by 12 weeks as demonstrated using CT Imaging, MRI
Imaging, non-destructive manual testing and histology. The
autologus treated levels achieved similar fusion rates but
demonstrated a slower progression taking upto 26 weeks.
However this success in
lower animals did not translate as such in the higher animals.
Martin et al demonstrated that the rh BMP 2 in a concentration
of 0.43 mg/ml that was effective in lower animals was not
effective in Rhesus monkey. It was hypothesized that overlying
muscle mass may have been the cause of mechanical compression of
the collagen sponge resulting in its splaying. This reduced the
local concentration of BMP at the site, consequently impeding
the bone formation.
In order to avoid the
problems associated with mechanical compression and splaying of
the collagen sponge alternative carriers were investigated.
These carriers unlike collagen sponge (that has a wet tissue
paper like consistency on getting soaked) were more compression
resistant and maintained higher concentration of BMP at the
local site. Boden et al developed a highly porous biphasic
calcium phosphate (BCP) ceramic carrier consisting of 60%
hydroxyl apatite and 40% tri calcium phosphate for use in
posterolateral fusions in primate. They were able to demonstrate
fusion was achieved at different rh BMP 2 concentrations (1.4,
2.1 and 2.8mg/ml) but was not achieved in any animal in which
auto graft was implanted.
Human studies: In view of
the findings obtained from the non-human primate studies,
different concentration and carrier media were investigated for
use in humans. Boden et al followed their primate study with a
pilot prospective randomized study in human subjects suffering
from a single level degenerative disc pathology and showed 100%
fusion rates with rh BMP2 in biphasic calcium phosphate (BCP)
carrier media as compared to only 40% fusion rates in the auto
grafted group.
In another human study,
Patel et al did a safety and efficacy study for use of OP-1 in
posterolateral spinal fusion. Sixteen patients suffering with
degenerative lumbar spondylolisthesis were randomized in to two
groups of autograft without instrumentation and autograft and
OP-1 without instrumentation. At 6 months, the autograft and
OP-1 showed a 75% fusion rate as compared to only 50% in the
autograft group alone. Clinical success as defined by
improvement of 20% or more of Oswestry score was seen in 83% of
auto grafted and OP-1 patients compared to only 50% in the auto
grafted patients.
At time of writing this
paper the BMP is still not approved for routine use in
posterolateral fusion. The use of OP 1 for revision fusion
surgeries is however allowed.
Non-Unions: Delayed
and non-unions have always remained a challenge for orthopedic
surgeons. The earliest use of BMP was done in femoral non-union
in 1988. Johnson et al. treated twelve patients with an
intractable femoral non-union and an average of 4.3 previous
attempts at surgical union, with internal fixation and partially
purified BMP extract. Eight of the twelve patients also received
either an autogenous or an allogenic bone graft. Eleven of the
twelve patients healed after this single intervention. The same
researchers later reproduced their results in tibial non unions
where in six of the patients with 3 –17 cm of tibial segmental
defect non union successfully healed after a single implantation
of purified BMP and autograft.
In 1992 the first FDA
approved investigational human clinical trial for evaluation of
OP 1 in treatment of tibial non union was started. 122 patients
with 124 established tibial non unions were randomized in two
groups, one group of 61 patients (61 non unions) were treated
with intramedullary rod and fresh autogenous bone graft, the
other group of 61 patients (63 non unions) was treated with
intramedullary rod and OP1. The patients were assessed at 3, 6,
9, 12 and 24 months for severity of pain at the fracture site,
ability to walk full weight bearing, need for second
intervention, and radiographic evaluation of bony union.
Clinically satisfactory outcome was observed in 81% of
population treated with OP 1 as compared to 85% of the auto
graft treated group. The OP1 group had significantly less blood
loss, a shorter hospital stay and a decreased operative time.
The radiographic evidence of union was established in 75% of OP
1 treated case as compared to 84% cases, the difference was
considered statistically insignificant (p=0.218).
Other clinical experiences
have confirmed the above evidence, In a study of 31 patients
with 6 tibial, 9 clavarial, 10 humeral, 2 ulnar and 4 femoral
non unions who under went standard internal fixation
supplemented with OP 1, McKee et al found abundant new bone
formation in all 31 patients and fracture healing at a mean of
13 weeks without any adverse clinical event in response to OP1.
A retrospective study of 12 humeral non unions treated with OP 1
Implant ™ by Susarala et al showed clinical and radiographic
evidence of union in 11 of the twelve patient at an average of
162 days.
Open Tibial Fractures:
In a randomized prospective study investigators who organized
themselves under BMP– 2 evaluation in surgery for tibial trauma
(BESTT) study group, reported the results of a study in 450
patients with open tibial fractures. 450 patients were
randomized into two groups: One group received a standard care
in form of initial irrigation and debridement followed by a
statically locked intramedullary nail. The second group received
either 0.75 or 1.50 mg/kg rh BMP 2 on an absorbable collagen
sponge at the time of wound closure.
58% patients treated with
1.50mg/kg rh BMP-2 healed compared to only 38% patients healed
in the group, treated with standard care (p=0.0008). Moreover
the patients who received rh BMP 2 had fewer hardware failures,
fewer infections and demonstrated faster wound healing.
At present times, the
indications of BMP use in fracture cases include: use of BMP 7
or OP1 in cases of recalcitrant non unions and BMP 2 in cases of
acute open tibial fractures.
Potential application
(still in investigational stage):
Intervertebral disc
repair: The use of BMP in affecting the intervertebral
repair had some encouraging results in animal models. Disc
defect models were created in rabbit- either using a lysis model
that involved injection of chondroitnase to disrupt the extra
cellular matrix or a needle puncture model. Aqueous rh BMP -7
(OP1) was injected in disc spaces four weeks after creation of
the defect. The animal were then followed over a period of three
months. A control disc defect group, which did not receive OP 1,
was followed for same period. After 3 months, while the disc
that received the OP –1 demonstrated a recovery of disc height
almost equivalent to original disc height, the control group
showed an approximate 40% reduction in the disc height. The
above findings suggested the possible etiopathologic and
therapeutic role of BMPs in disc degeneration and repair.
Role in total joint
arthroplasty: Cortical perforations, bone loss, acetabular
defects, and periprosthetic fractures are some of the challenges
that total joint replacement surgeons encounter today. Auto
grafts or in some instances allograft are currently used to
tackle these complex issues. Studies have shown that healing of
cortical strut graft to femur is significantly increased with
addition of OP 1. These findings are significant in view of the
fact that autograft when used alone, although leads to healing
of the defects does not necessarily leads to boney in growth.
The use of allograft that appears to be an attractive
alternative suffers form lack of the osteoinductive properties
of auto graft or BMP.
Osseo integration of
prosthetic devices: The studies that have investigated the
effect of BMP as direct coating on implants or in conjunction
with carrier material have shown that BMP can promote Osseo
integration at the implant bone interface.
Distraction
Histogenesis: Nature of regenerate in Illizarov technique
for treating non unions have a significant bearing on the final
out come. The use of BMP 2 and OP 1 (BMP7) as injection at the
distraction site prior to distraction and during consolidation
phase is being currently evaluated.
Osteonecrosis of femoral
head: Present treatment of avascular necrosis or
osteonecrosis of femoral head includes core decompression, bone
grafting and in later stages hip arthroplasty. Ongoing research
is studying the possible role of implantation of BMP after
removal of the necrotic core, either alone or in combination
with other growth factors.
Repair of articular
cartilage with OP 1: Articular cartilages have a limited
potential for repair and following injury or degeneration,
secondary procedures like arthroplasty are required to maintain
joint congruity. BMP 7 and BMP 2 have shown promise in repair of
full thick osteochondral cartilage defects in animal models. A
study designed to evaluate the effect of OP-1 on full thickness
osteochondral defects in sixty five mongrel dogs demonstrated
that implantation of rh BMP 7 into full thickness articular
cartilages improved the histologic appearance of the repair
tissue compared with that of both untreated and collagen carrier
control defects.
Beyond bones - effect of
BMP on joint development, articular cartilage, kidney
development, neural tissues, and cancer:
BMP name can at best be
considered misleading for this group of molecules that have
shown to regulate biological processes as diverse as cell
proliferation, apoptosis, cell differentiation, cell fate
determination and morphogenesis. BMPs have effect on development
of all three germ layers, and thus the development of nearly all
organs and tissues. It helps orchestrating a well-executed basic
embryonic body plan.
BMPs are potent morphogens,
capable of inducing mesenchymal cells into chondrocytes. The
earliest studies involving the rabbit knee to study regeneration
of artificially created osteochondral defect have shown an
accelerated formation of subchondral bone and improved cartilage
formation82.
In view of its multipotent role in nearly all the aspects of
development and regeneration, its role is aggressively being
tested for clinical application in multitude of fields beyond
orthopaedics. One of the most interesting fields where their use
has potential of revolutionizing the management is its use in
management of nephropathies. BMP 7 has shown to partially
reverse diabetic-induced kidney hypertrophy, restore GFR, urine
albumin excretion, and glomerular histology toward normal.
Restoration of BMP-7 expression has been shown to be associated
with a successful repair reaction and a reversal of the
ill-fated injury response. Another recent study on role of BMP
in reversing renal damage has suggested that adult renal
fibroblasts might retain parts of their original embryonic
imprint and plasticity, which can be re-engaged by systemic
administration of BMP-7 to mediate repair of tubular injury in a
fibrotic kidney.
Conclusions
After decades of intense
research BMPs have finally moved from the realm of in vitro to
in vivo. BMPs have demonstrated beyond doubt their role as a
superior alternative of autogenous bone graft. Recent research
has instilled renewed hopes of its use in varied musculoskeltal
conditions including disc regeneration, cartilage repair,
osteonecrosis and hip arthroplasties. Progress in delivery
materials and techniques may further its use in minimally
invasive procedures which will decrease not only the operative
time, but also decrease the overall morbidity and recovery time.
Amidst this enthusiasm of
use of BMP in varied clinical situations, some studies however
sound words of caution. Much of the data in BMP research has
been derived from animal studies which are important as far as
providing base line data for further clinical studies is
concerned, but it would be prudent not extrapolate data as it is
to humans. The rate of bone repair is inversely related to the
position of a species on the phylogenetic scale so that there is
decreased potential of bone formation in humans and higher
primates when compared to lower animals like mice and rabbits,
moreover the quadrepeds have different biomechanics compared to
upright humans. A host of other factors including smoking, age,
steroid use, osteoporosis, malnutrition, disease severity play a
role in determining the physiology of bone regeneration in
humans. Thus the true efficacy and safety of these agents for
different scenarios must be established in carefully designed
prospective randomized clinical trials before they are approved
for use.
On the sunny side, the
impact of the discovery and progress in this field can be gauged
by the fact that its use as bone graft substitute alone has
potential to replace the autogenous bone graft in 1 million
procedures that are performed world wide every year. The
potential for its use in other sectors is still untapped and the
actual impact of discovery will be fully known only after some
years. A disconcerting issue however is the cost of BMP.
Currently the estimated cost of BMP at 3000$ to 5000$ limit
their clinical use to selected clinical scenarios like
recalcitrant non-union and revision fusion surgeries, it is
however hoped that the cost drops and BMP eventually becomes as
affordable as other recombinant products like recombinant
insulin or recombinant vaccine, enabling its use in majority of
indicated patient population.
In a nutshell it is time
for orthopedic surgeons to look beyond just the bone and metals
and embrace newer technologies that involve manipulation of
cellular environment to achieve the desired bone formation and
BMP may just be the road ahead.
References
1. Arrington ED SW,
Chambers HG, et al. Complications of illiac crest bone graft
harvesting. Clin Orthop 329:300-309, 1996.
2.Banwart JC AM, HAssanein RS. Iliac crest bone graft harvest
donor site morbidity: A statistical evaluation. Spine
20:1055-1060, 1995.
3. Fernyhough JC SJ, Weigel MC, et al. Chronic donor site pain
complicating bone graft harvesting from posterior illiac crest
for spinal fusion. Spine 17:1474-1480, 1992.
4. Laurie SWS KL, Mulliken JB, et al:. Donor site morbidity
after harvesting rib and illiac bone. Plast Reconstr Surg
73:933-938, 1984.
5. Schnee CL FA, Weil RJ, et al:. Analysis of harvest morbidity
and radiographic outcome using autograft for anterior cervical
fusion. Spine 22:2222-2227, 1997.
6. Urist M. Bone Formation by osteoinduction. Science
150:893-899, 1965.
7. Sampath TRA. Dissociative extraction and reconstitution of
extracellular matrix composition involved in local bone
differentiation. Proc Natl Acad Sci 78:7599-7602, 1981.
8. Wang ERV, Cordes P, Hewick R, Kriz M, Luxenberg D, Sibley B,
Wozney . Purification and characterisation of other distinct
bone inducing proteins. Proc Natl Acad Sci 85:9484-9488, 1998.
9. Celeste AIJ, Taylor R. Hewick R, Rosen V, Wag E, Wozney J.
Identification of transforming growth factor beta superfamily
members present in bone inductive protein purified from bovine
bone. Proc Natl Acad Sci 87:9843-9847, 1990.
10. Sampath TRA. Homology of bone inductive proteins from
humans, monkey, bovine and rat extracellular matrix. Proc Natl
Acad Sci 80:6591-6595, 1983.
11. Sampath T, Rashka K, Doctor J, Tucker R, Hoffman F.
Drosophilia TGF beta superfamily proteins induced enchondral
bone formation in mammals. Proc Natl Acad Sci 90:6004-6008,
1993.
12. Urist MDR, Finerman G. Bone cell differentiation and growth
factors. Science 220:680-686, 1983.
13. Tsuda TMK, Yoshikawa H, Shimuzu N, Takaoka K.
Establishment of an osteoinductive murine osteosarcoma clonal
cell line showing osteoblastic phenotypic traits. Bone
10:195-200, 1989.
14. Takoka KYH, Masuhara K, Sugamoto K, Tsuda T, Aoki Y, Ono Y,
Sakamoto Y. Establishment of a cell line producing BMP from a
human osteosarcoma. Clin Orthop 244:258-264, 1989.
15. Urist MMA, Lietze A. A solubilized and insolubilized bone
marphogenic protein. Proc Natl Acad Sci 76:1828-1832, 1979.
16. Bauer FUM. Human osteosarcoma derived soluble bone
morphogenic protein. Clin Orthop 19811981.
17. Reddi A. Bone Morphogenic proteins: From basic science to
clinical applications. JBJS 830 - A:1-6, 2001.
18. Griffith D, Keck PC; Sampath Tk; Rueger DC; Arlson Wd.
Three dimensional structure of recombinant human osteogenic
protein 1: Structural pradigam for the transforming growth
factor beta superfamily. Proc Natl Acad Sci 931996.
19. Massague J. TGF -beta signal transduction. Annu Rev Biochem
67:753-791, 1998.
20. Kingsley DM. The TGF-beta superfamily : new members, new
receptors, and new genetic tests of function in different
organisms. Genes Dev 8:133-146, 1994.
21. Wozney J. The bone morphogenetic protein family:
multifunctional cellular regulators in the embryo and adult. Eur
J oral sci 106:160-166, 1998.
22. Franceschi R. The
developmental control of osteoblast specific gene
expression:role of specific transcription factors and the
extracellular matrix environment. Crit Rev Oral biol Med
10:40-57, 1999.
23. Nishida YKC, Eger
W, Kuettner KE, Knudson W. Osteogenic protein 1 stimulates cell
associated matrixassembly by normal human articular chondrocytes
up regulation of hyaluronan synthase, CD 44, and aggrecan.
Arthritis Rheum 43:206-214, 2000.
24. Nishida YKC, Eger
W, Kuettner KE, Knudson W. Osteogenic Protein 1 promotes the
synthesis ad retention of extracellular matrix within bovine
articular cartilage and chondrocyte cultures. Osteoarthritis
Cartilage. 8:127-136, 2000.
25. Reddi A. Bone and
cartilage differentiation. Curr opin genet dev 4:737-744, 1994.
26. Reddi A.
Cartilage morphogenesis: role of bone and cartilage
morphogenetic proteins, homeobox genes and extracellular matrix.
Matrix Biol 14:599-606, 1995.
27. Reddi A. Role of
morphogenetic proteis in skeltal tissue engineering and
regeneration. Nat Biotechnol 16:247-252, 1998.
28. Reddi A.
Morphogenetic messages are in extracellular matrix:
biotechnology from bench to edside. Biochem soc tras 28:345-349,
2000.
29. Reddi A. Bone
morphogenetic proteins and skeltal dvelopment: the kidney - bone
connection. Pediatr Nephrol 14:598-601, 2000.
30. Hogan B. Bone morphogenic proteins: multifunctional regulators of vertebrate
development. Gees Dev 10:1580-1594, 1996.
31. Hogan B. Bone
Morphogenetic proteins in development. Curr opin genet dev
6:432-438, 1996.
32. Sampath TRD.
Structure, function and orthopedic applications of osteogenic
protein -1. Complications in Orthopedics 9 (winter):101-107,
1994.
33. Wozney JM RV,
Bone morphogenic proteins and their gene expression., in in
Cellular and molecular biology, M. Noda, Editor. 1993, Academic
press: San Deigo. p. 131-167.
34. Blokhuis tdoFBJ,
Jenner J , Bakker F, Patka P, Haarman H. Biomechanical and
histological aspects of fracture healing, stimulated with
osteogenic protein-1. Biomaterials 22:725-730, 2001.
35. Ashley RLJ.
Safety profile for the clinical use of bone morphogenetic
protein in the spine. Spine 28:372-377, 2002.
36. Soda H RE, Sharma
S, et al. Anti proliferative effects of recombinant human bone
morphogenic protein-2 on human tumor colony forming units.
Anticancer Drugs 9:327-331, 1998.
37. Orui H IS, Ogino
T, et al. Effects of Bone morphogenic protein 2 on human tumor
cell growth and differentiation: a preliminary report. J Orthop
Sci 5:600-604, 2000.
38. Poynton AR LJ.
Safety profile for clinical use of bone morphogenic protein in
the spine. Spine 27:40-48, 2002.
39. Burkus JGMDCZT.
Anterior interbody fusion using rh BMP2 with tapered cages. J
Spinal Disorders 15:337-349, 2002.
40. Paramore CG LC,
Rauzzino J, et al. The safety of OP-1 for lumbar fusion with
decompression: A cnaine study. Neurosurgery 44:1151-1156, 1999.
41. Boden SMG, Morone
MA, etal. Posterolateral Lumbar intertransverse process spine
arthrodesis with recombinant human bone morphogenic protein -2/
hydroxyappatite tri calcium phosphate after laminectomy in the
non human primate. Spine 24:1179-1185, 1999.
42. Alexander JT BCJ,
Haid RW Jr, Rodts GE, Subach BR, Shaffrey CL. An analysis of the
use of rh BMP2 in PLIF constructs: clinical and radiolographic
outcomes. Presented at the AANS/ CNS section on Disorders of
Spine and Peripheral nerves 2002, Feb 27- Mar 2;2002.
43. David SM MT,
Tabor OB, et al. Lumbar spinal fusion using recombinant human
bone morphogenic protein in the canine: A randomized, blinded
and controlled study. Trans Int Soc Study Lumbar Spine 22:14,
1995.
44. Boden SD KJ,
Sandhu HS, Heller Jg. Use of rh BMP2 to achieve posterolateral
lumbar spine fusion in humans: a prospective randomized clinical
trial. Spine 27:2662-2673, 2002.
45. Yasko A LJ,
Fellinger E, Rosen V, Wozney J, Wang E. The healing of segmental
defects induced by recombinant human bone morphogenic protein (rh
BMP2). Journal of Bone & Joint Surgery American 74 A:59- 67,
1992.
46. Cook S BG, Wolfe
M, Sampath T, Rueger D, Whitecloud T. The effect of recombinant
human osteogenic protein -1 on healing of large segmental bone
defects. Journal of Bone & Joint Surgery American 76A:278-238.,
1994.
47. Bostorm M LJ,
Tomin E, Browne M, Berberian W, Turek T, Smith J, Wozney J,
Schidhauer T. use of bone morphogenic protein -2 in the rabbit
ulnar non union model. Clin Orthop and related research.
327:272-282., 1996.
48. Gerhart T KHC,
Kriz M. Healing segmental femoral defects in sheep using
recombinant human bone morphogenic protein. Clin Orthop
293:317-326, 1993.
49. Reddi A. Fracture
repair process: Initation of fracture repair by bone
morphogenetic proteins. Clin Orthop and related research.
355S:S66-72, 1998.
50. Sandhu HKLTAe.
Augmentation of Titanium fusion cages for experimental anterior
lumbar fusion. NASS 1996:47, 1996.
51. Diwan ASH, Kanim
LEA, etal. Histological evaluation of the efficacy of rh BMP2
when compared to autogenous bone in sheep spinal interbody
fusion using a titanium cage. NASS 2000:135-136, 2000.
52. Zdeblick TGA,
Rapoff AJ, et al. Cervical interbody fusion cage: An animal
model with and without bone morphogenic protein. Spine
23:758-766, 1998.
53. Boden SMG, Horton
WC et al. Laparoscopic anterior spinal arthrodesis with rh BMP 2
in titanium interbody threaded cage. J Spinal Disorders 111998.
54. Gornet MF Bk,
Dickman CA. rh BMP 2 with tapered cages: A prospective,
randomized Lumbar fusion study. Read at the annual meeting of
the North American Spine Soceity; Oct 31-Nov 3; Seattle, WA2001.
55. Burkus JTE,
Kitchel SH, Watkins Rg, Balderston RA. Clinical and radiographic
outcomes of anterior lumbar interbody fusion using recombinant
human bone morphogenic protein- 2. Spine 27:2396-2408, 2002.
56. Magin M DG.
Improved lumbar vertebral interbody fusion using rh OP1. Spine
26:469-478, 2001.
57. Chirossel JP GE,
Boutrand JP, et al:. Stryker spine PEEK and titanium cages:
Interbody fusion with OP-1vs autograft in a sheep model.
Presented at Eurospine, Antwerp, Belgium. 2000.
58. Baskin DS RP,
West mark R, Lynch J, Bartolomei J, Theodore N, Sonntag VKH.
ACDF with cornerstone SR allograft and plate: rh BMP 2 vs
autograft. Presented at the AANS/ CNS section on Disorders of
Spine and Peripheral nerves Feb 27- Mar 2 2002, Orlando
Florida.2002.
59. Boden SMP, Morone
MA, et al. Video assisted lateral intertransverse process
arthrodesis: validation of a new minimally invasive lumbar
spinal fusion technique in the rabbit and non human primate
(rhesus) model. Spine 21:2689-2697, 1996.
60. Suh D, Boden SD,
Ugbo JL, etal. Use of rh BMP 2 supplemented with allograft chips
in posterolateral fusions in non human primates. AAOS 2002:70,
2002.
61. Schimandle JH,
Boden SD, Hutton WC. Experimental spinal fusion with recombinant
human bone morphogenetic protein-2. Spine 20:1326-1337, 1995.
62. Sandhu HS KLTJea.
Experimental spinal fusion with recombinant Bone morphogenic
protein 2 without decortication of osseous elements. Spine
22:1171-1180, 1997.
63. Cook SD DJ, Tan
EH, et al:. In vivo evaluation of recombinant human osteogenic
protein (rh OP1) implants as bone graft substitute for spinal
fusion. Spine 19:1655-1663, 1994.
64. Martin GJ, Jr.,
Boden SD, Marone MA, et al. Posterolateral intertransverse
process spinal arthrodesis with rhBMP-2 in a nonhuman primate:
important lessons learned regarding dose, carrier, and safety.
Journal of Spinal Disorders 12:179-186, 1999.
65. Boden SD, Martin
GJ, Jr., Morone MA, et al. Posterolateral lumbar intertransverse
process spine arthrodesis with recombinant human bone
morphogenetic protein 2/hydroxyapatite-tricalcium phosphate
after laminectomy in the nonhuman primate. Spine 24:1179-1185,
1179.
66. Patel Tc VA,
Truummess E, et al. A safety and efficacy study of OP-1 as an
adjunct to posterolateral Lumbar fusion. Presented at th North
American Spine Society Meeting, Seattle, Washington. 2001.
67. Johnson EE, Urist
MR, Finerman GA. Bone morphogenetic protein augmentation
grafting of resistant femoral nonunions. A preliminary report.
Clinical Orthopaedics & Related Research 230:257-265, 1988.
68. Johnson EE, Urist
MR, Finerman GA. Distal metaphyseal tibial nonunion. Deformity
and bone loss treated by open reduction, internal fixation, and
human bone morphogenetic protein (hBMP). Clinical Orthopaedics &
Related Research 250:234-240, 1990.
69. Friedlaender GE
PC, Cole JD, Cook SD, Cierney G, Muschler GF, Zych GA, Calhoun
JH, La forte AJ, Yin S. Osteogenic protein -1 in the treatment
of tibial non union. Journal of Bone & Joint Surgery American 83
(Suppl 1):S 151-158, 2001.
70. Mckee MD SE,
Waddell JP, Wild L. The treatment of long bone non union with rh
BMP: results of a prospective pilot study. Poster presentation
#242, 71st AAOS meeting, 10 -14 March, San Francisco, CA. 2004.
71. Susarla A LF,
Tejwani NC, Koval KJ, Egol KA. OP 1 implant as an adjunct to
mechanical fixation in humeral non union. Poster presentation
#241, AAOS meeting 10 -14 march, San Francisco, CA. 2004.
72. Govender S,
Csimma C, Genant HK, et al. Recombinant Human Bone Morphogenetic
Protein-2 for Treatment of Open Tibial Fractures: A Prospective,
Controlled, Randomized Study of Four Hundred and Fifty Patients.
J Bone Joint Surg Am 84:2123-2134, 2002.
73. Masuda K IY,
Okuma M, Nakagawa K, Akeda K, Muehelman C, Thonar E, Andersson
G, AN H>. Osteogenic protein -1 injection into a degenerated
disc induced a significant regeneration of the intervertebral
disc in the rabbit annular puncture model. NASS 2004 19th annual
meeting2004.
74. Howard AS TK,
Kamada H, Nguyen CM, Thonar EJ, Singh K, Andersson GB, Masuda
K:. Intradiscal administration of Osteogenic protein -1 increase
intervertebral disc height and proteoglycan content in nucleus
pulposus in normal adolescent rabbits. Spine 302005.
75. Jensen TB OS,
Lind M, Rahbek O, Bunger C, Soballe K. Osteogenic protein 1
device increases bone formation and bone graft resorption around
cementless implants. Acta Orthop Scand 73:31-39, 2002.
76. Lind M OS, Song
Y, Goodman S, Bunger C, Soballe K. Osteogenic protein 1 device
stimulates bone healing to hydroxy appatite coated and titanium
implants. J Arthroplasty 15:339-346., 2000.
77. Lind M OS, Jensen
T, Song Y Goodman S, Bunger C, Soballe K. Effect of osteogenic
protein 1/ collagen composite combined with impacted alllograft
around hydroxy appatite coated titanium alloy implants is
moderate. J Biomedical Mat Res 55:89-95, 201.
78. Lind M, Overgaard
S, Song Y, et al. Osteogenic protein 1 device stimulates bone
healing to hydroxyapaptite-coated and titanium implants. Journal
of Arthroplasty 15:339-346, 2000.
79. Amako M HR,
Steffen T. The effects of a single injection of OP1 on
stimulating new bone formation in distraction osteogenesis in
the rabbit. Abstract in 47th annual meeting , Orthopedic
Research society, San Francisco, CA 26:146., 2001.
80. Cook SD, Patron
LP, Salkeld SL, et al. Repair of Articular Cartilage Defects
with Osteogenic Protein-1 (BMP-7) in Dogs. J Bone Joint Surg Am
85:116-123, 2003.
81. BLM H. Bone
mOrphogenic proteins- multifunctional regulators of vertebrate
development. Gen Develop. 10:1580-1594, 1996.
82. Sellers RC ZR,
Glasson SS, Kim HD, Peluso D, D'Augusta DA, Beckwith K, Morris
EA. Repair of articular cartilage defects one year after
ytreatment with bone morphogenic protein -2 (rh BMP2). Journal
of Bone & Joint Surgery American 82- A:151-160, 2000.
83. Wang S CQ, Simon
TC, Strebeck F, Chaudhary L, Morrissey J, Liapis H, Klahr S,
Hruska KA. Bone morphogenic protein-7 (BMP-7), a novel therapy
for diabetic nephropathy. Kidney Int. . 63:2037-2049, 2003.
84. Zeisberg M SA,
Kalluri R. Bone morphogenic protein-7 induces mesenchymal to
epithelial transition in adult renal fibroblasts and facilitates
regeneration of injured kidney. J Biol Chem. 280:8094-8100,
2005.
|



