|
Abstract:
By the time an injury occurs to the articular cartilage, the capacity that it shows for self-repair is very limited. Surgical therapeutic procedures to cartilage repair exist and are clinically useful, but, in spite the progress that has been achieved, they cannot restore a normal articular surface. That is why current research focuses on a growing number of bioactive reagents that may act and modify the repair process. As these agents consist mainly of proteins and nucleic acids, it is difficult to administer them effectively. Consequently, gene transfer approaches are being developed, so that these agents are synthesized at the appropriate sites.
Regeneration of articular cartilage can be obtained through the delivery of therapeutic genes to the synovium, or directly to the cartilage lesion.
The cells of the synovial lining are generally preferred as target cells for the chondroprotective approaches, based on the expression of anti- inflammatory mediators. In the rest of the cases, where the target is the cartilage defect, gene transfer can be achieved by either direct vector administration to cells located at or surrounding the defects, or by transplantation of genetically modified chondrogenic cells into the defect. It has been shown that local delivery of exogenous cDNAs encoding growth factors to sites of cartilage damage is possible. Furthermore, the growth factors are expressed at therapeutical levels at these sites. The most interesting point is that a more hyaline-alike cartilage repair tissue is synthesized when adequate levels of gene expression are achieved. This review presents the current status of gene therapy for cartilage healing and demonstrates its potential role in osteoarthritis therapy.
J.Orthopaedics 2012;9(1)e7
Keywords:
Osteoarthritis; articular cartilage; gene therapy; chondrocyte; mesenchymal stem cell; synovial cell; growth factor
Introduction:
When considering tissue engineering, it could be easily assumed that cartilage would be one of the easiest tissues to target, as two major obstacles, namely angiogenesis and creation of multilayered tissues derived from cells of multiple lineages, do not exist. The cartilage has some almost unique properties, being avascular and composed of a single cell type, the chondrocyte. Despite this, the reality is that in fact, very few clinical trials have taken place, following successful animal models, for cartilage regeneration. The result of this is that the conservative treatment of disorders like osteoarthritis continues to rely mostly on analgesics or on therapies that their efficacy is still argued, whereas the surgical interventions, given the limited regenerative capacity of cartilage, at best, temporarily alleviate some of the symptoms without addressing the underlying pathology, until the final solution of total joint arthroplasty takes place.
By recognizing the serious limitations of the conservative treatment and the insufficiency of the surgical interventions to treat appropriately these disorders, the first attempt was to incorporate cell transplantation as a treatment option, as it has been clinically proven to give better long term results [1,2].
The effectiveness of these treatments is being enhanced thanks to tissue engineering research, by promoting the use of growth factors and cytokines in order to regenerate tissue that more accurately resembles native cartilage. Apart from this, there is also the possibility of direct intervention to the synovial cells or the chondrocytes of the joint that suffers from osteoarthritis.
A variety of biological factors has been identified and proved to reduce inflammation or promote regeneration. What is really challenging now, is the development of the appropriate delivery systems, which will be able to deal with the complexity in delivery of biological agents. The direct delivery of these agents, either systemically or locally, is almost impossible as they have a very short half life (minutes), which means that very frequent administration at high doses is needed. Another fact that should not be ignored is that therapeutic concentrations for one organ may be harmful to another. These problems can be solved by creating genetically modified cells, able to transiently over-express certain proteins. This procedure can be done either ex vivo or in vivo. Each way possesses its own advantages and disadvantages which will be discussed later on in this review. Though gene therapy for cartilage regeneration is still in an early stage, it can be expected that in the near future multiple cell populations will be available, each expressing a different protein of interest, working in synergy to control inflammation and encourage regeneration. This review article is going to present the limitations and the potential of gene delivery for cartilage regeneration.
Cartilage injury:
limitations of current treatments
Hyaline articular cartilage is a highly specialized tissue that protects the bones at the area of joints from forces associated with load bearing, friction and impact. For this reason it is a remarkably durable tissue, but, once been injured, its self-repair is almost impossible. In partial thickness defects, there is no involvement of the vasculature, which means that chondroprogenitor cells cannot enter the damaged region and participate in the reparative process. Articular chondrocytes do not participate also, being unable to migrate to the lesion. All these lead to the permanent remaining of such defects [3,4]. On the other hand, full thickness cartilage injuries cause rupture of blood vessels and hematoma formation at the injury site, which initiates a repair response, resulting in the formation of a fibrocartilage repair tissue within weeks [3,4]. This is the basis of marrow stimulating surgical techniques, such as abrasion arthroplasty or microfracture, which aim to promote a natural fibrocartilaginous response in focal cartilage defects [5-9]. One limitation of these procedures is the fact that fibrocartilage has inferior mechanical and biochemical characteristics compared to normal hyaline articular cartilage, as it is poorly organized, contains significant amounts of collagen type I and is susceptible to injury. With time and repeated loading fibrocartilage destruction is inevitable and premature OA occurs [3,4].
Therefore, the aim of modern therapeutic techniques is to achieve a more hyaline- like cartilage repair tissue by transplanting tissues or cells. Osteochondral transplantation procedures have shown positive short term results, but the long term clinical results are uncertain. Apart from this, the biggest limitation is the tissue availability for transplant, especially in large cartilage defects [4,5,10-12].
Therefore, the autologous chondrocyte transplantation procedure has been introduced, combined with a periosteal cover to treat chondral or osteochondral defects of the knee with good clinical results [6,13-15]. Despite this, most surgical interventions only result in improvement of clinical symptoms, as the regeneration of hyaline cartilage tissue is not yet achieved [4,5,14,16]. This is the reason why efforts are made to engineer cartilage in vitro, in order to produce grafts that will facilitate regeneration of articular cartilage in vivo. However, no significant improvement has occurred in this domain when compared to current cartilage repair procedures and many challenges remain for the successful formation of hyaline repair tissue in vivo [4,11,17,18].
Candidate cells for gene delivery
Cartilage is classified into three main categories elastic cartilage found in the external ear, fibrocartilage in the intervertebral discs and hyaline cartilage which is present at the articular surfaces and represents the most abundant type of cartilage. This type of cartilage is affected in arthritic disorders like osteoarthritis and this is the reason that most tissue engineering models focus on regeneration of articular cartilage, which is a type of hyaline cartilage. Chondrocytes produce the extracellular matrix of the hyaline cartilage and get immobilized there, occupying only 2% of the total volume. The result of this fact is that the mechanical and biochemical properties of the cartilage are defined by its extracellular matrix.
1.Autologous chondrocyte
s
As chondrocytes are the cells that secrete extracellular cartilaginous matrix that defines the cartilage, this type of cells seems to be the most appropriate to choose for gene delivery at first view. Unfortunately, these cells are inaccessible to vectors due to the rich matrix that surrounds them, so gene delivery approaches for tissue engineering of cartilage seldom target the chondrocytes in vivo. Instead, autologous chondrocytes harvested from joints and expanded in vitro in monolayers are preferably used for transfection. Adenoviral-mediated delivery of various transgenes, such as TGF-ί1, BMP-2, IGF-1 or BMP-7, has been shown to stimulate the production of cartilage specific matrix rich in collagen type II and proteoglycans, and, at the same time, to decrease tendency towards dedifferentiation [19-23]. Transfer of cDNA encoding matrix molecules, such as the collagen type II mini-gene, led to enhanced extracellular matrix production of human fetal chondrocytes [24]. Transduction with the transcription factor SOX-9 increased collagen type II expression of chondrocytes in three-dimensional culture in vitro [25,26], whereas overexpression of the transcription factor Runx-2 (Cbfa-1) stimulated chondrocyte maturation and induced a hypertrophic phenotype, expressing high levels of collagen types II and X, alkaline phosphatase and osteogenic marker genes [27,28].
Although the results of the previously mentioned studies might be promising, there are also some major obstacles. A serious limitation in the use of autologous cells is that they lose their chondrocytic phenotype and become fibroblastic when grown in vitro in monolayer after the first passage of cells [29,30]. This means that the quantity of cells that is available for transfection and implantation is severely limited, let alone that, in arthritic joints, this population of chondrocytes is already limited. As cartilage is an aneural tissue, initial damage is not perceived by the patient. Only when the patient starts to feel pain and gets immobilized he does seek medical advice. By this time, the damage is already significant, as there is more generalized cartilage loss that has reached subchondral bone. But, apart from this, even during the very early stages of osteoarthritis, there is a significant change in the phenotype of the chondrocytes. Different chondrocyte surface markers are expressed and an accelerated production of extracellular matrix molecules occurs, in an attempt to cope with the progressing damage [31]. Gradually the cell fails to respond to the anabolic demands of the damaged tissue and the chondrocytes undergo apoptosis, resulting to the loss of cartilage. Considering that the affected chondrocytes cannot keep up with the existing anabolic rhythms of the damaged cartilage, it would be unrealistic to force them to express additional genes.
2.Mesenchymal stem cells
On the other hand, mesenchymal stem cells (MSCs) are increasingly being investigated as better alternatives for cell transplantation, compared to autologous chondrocytes. Adequate quantities of autologous MSCs can be obtained by minimally invasive techniques from the iliac crest of the patient. Other populations of MSCs are found also in blood and in the periosteum. MSCs are multipotent cells, which means that they have the capacity to differentiate to numerous cell lines, including chondrocytes, although this potential varies, depending on the origin of the MSCs.
The first study to investigate the use of MSCs in this domain used bone marrow derived MSCs from New Zealand white rabbits for cartilage regeneration in full thickness cartilage defects [32]. Collagen sponges were used as scaffold for the MSCs that were then embedded into the defect. Under these conditions, MSCs secreted a cartilaginous matrix- and this matrix was converted to bone in subchondral regions of the defect- even without using additional growth factors. What was shown by this study, is that the use of differentiated chondrocytes is not mandatory for cartilage regeneration. What should moderate the enthusiasm from this study is that the regenerated cartilage showed discontinuity with the existing cartilage and its mechanical properties were inferior compared to the properties of hyaline cartilage [33].
After this success, following studies recognized the need to stimulate MSCs with appropriate chondrogenic factors. Various growth factors such as BMP-2, BMP-7, IGF-1 and recombinant proteins led to better results at regeneration than the simple introduction of MSCs into the joint space. It should be kept in mind, however, that the environment in most of the experimental models does not resemble the environment of a damaged cartilage [34]. This means that the determination of the chondrogenic factors that are required to lead to true differentiation of the MSCs involves more work and is far more complex. Firstly, apart from the MSCs that are artificially introduced, there are also damaged chondrocytes and inflammatory cells inside the joint which all interact with each other, processes that are absent during normal development, when the already mentioned growth factors act. For example, IGF-1 in developing chondrocytes promotes the synthesis of cartilage specific matrix molecules [35]. However, when inflammatory cells are present, the expression of IGF-1 receptors is downregulated leading to an inferior response to this growth factor, compared to the expected one [36]. Apart from that, different animal models respond differently to the same biological factors. An example is the fate of collagen sponges embedded with recombinant BMP-2 and rabbit MSCs when implanted in rabbit knees, in comparison to similar sponges containing BMP-2 transfected perichondral cells that were implanted in the knees of rats. In the first case, the regenerated cartilage was thin and irregular [37], whereas in the second case the resultant cartilage had a rich collagen I component similar to fibrocartilage [38]. This example more probably reflects the complexities of research and its applications in tissue engineering, rather than the existence of any utility of MSCs as candidates for cartilage regeneration. In another experimental model, genetically modified periosteal cells transduced to express sonic hedgehog (SHH) were compared to the delivery of the BMP-7 cDNA, which resulted in a better overall repair of the SHH compared with the BMP-7 treated defects after 12 weeks postoperatively, and both were superior to marker gene controls [39]. Using the same animal model, constructs of a collagen type I hydrogel and marrow derived MSCs following liposomal GDF-5 (CDMP-1) gene delivery were shown to enhance cartilage repair compared with marker gene controls [40].
In conclusion, it should be kept in mind that MSCs have trivial disadvantages, the most important being the inhibition of T-cell proliferation through immunosuppressive effects [41]. Although this delays immune rejection of transplanted MSCs, so they can be used as allografts, it also causes skepticism when considering the possibility of tumor induction. Even though such events are rare, an extensive investigation is needed to further address this topic [42].
3.Cells within the synovial cavit
y
Synovial cells that line the joint cavity make up for the other cell type that is considered to be a promising alternative for gene therapy. Its advantage is that the synovium lines the internal surfaces of the joint cavity and has a large cell population covering a significant surface area. This tissue forms a good candidate target either for direct gene delivery or for injection of transplanted cells into the synovial cavity, as cell engraftment and transfection is exclusively observed in the synovial lining and can be well predicted [43]. The interesting thing is that, by this way, gene delivery can be simplified to an intra-articular injection of gene delivery vectors or cells [44]. A promising finding of studies examining gene transfer to synovial cells was that these cells continue to express significant amounts of gene product in vivo for at least 42 days after being transfected by lentiviruses [45]. What really caused optimism concerning gene transfer to synovial cells was the advanced response of these cells in clinical trials, compared to other models. For example, in a trial that examined intra-articular delivery of IL-RA via gene therapy, it was found that its anti-arthritic effect was 104 times more potent than the effect of the recombinant protein been delivered systematically [46]. A finding of this study that caused skepticism was that significant amounts of IL-RA were reported in peripheral blood and in major organs, probably because of the direct injection of lentiviruses into the joint, as this was not the point in older studies, when already transfected cells (ex vivo) where introduced to the joint [47]. Apart from IL-RA, other plasmids have been successfully examined by ex vivo gene transfer, including IGF-1 and TGF-ί. However, early enthusiasm was moderated by the findings of various studies that concluded that either ex vivo or direct gene transfer to synovial cells can be toxic and can result in joint fibrosis, osteophyte formation, extensive uncontrolled cartilage growth (in the presence of BMP-2), joint swelling and in some cases cartilage degeneration [44,48,49]. Considering these results in the context of cartilage repair, synovial gene transfer may be more suitable for delivering chondroprotective agents rather than strong anabolic transgenes with pleiotropic effects of their products. Many anti-inflammatory cytokines have this property
Candidate genes
Potentially, a big number of genes can be used in gene therapy of osteoarthritis and this gets even more complex as there are, as mentioned above, three types of cells that can be targeted. The complexity increases if we take in mind that there might be gene combinations, apart from simple genes, that could enhance chondrogenesis. It should be considered, also, that these genes may act differently when being in an inflammatory environment.
Transforming growth factor ί
Transforming growth factor (TGF-ί) superfamily, including TGF-ί1, which is responsible for initial cellcel
l interaction between condensing progenitor cells, includes genes that are commonly used for cartilage formation [50]. In inflamed joints, TGF-ί1 has anti-inflammatory properties and stimulates new matrix synthesis by chondrocytes [51,52]. TGF-ί2, also, regulates Indian hedgehog (Ihh) and Parathyroid Growth Hormone (PTHrP) expression and, by this way, mediates hypertrophic differentiation of chondrocytes [53]. However, it has not been used as extensively as TGF-ί1 for gene delivery. Another role of TGF-ί1-3 is the inhibition of the formation of angiogenesis in cartilage during development [54]. In an experimental model, a monolayer of intervertebral disc cells was transfected with TGF-ί1 cDNA and was grown in 3 dimensional pellet cultures. When compared to pellets containing cells not transfected with TGF-ί1 a 375475% increase in proteoglycan synthesis was found [55]. On the other hand, irrespective of the number of cells used to grow these pellets, a diameter of more than 5-7 mm could not be achieved. A larger size of pellets could probably be achieved under the influence of different genes or of a combination including TGF-ί1.
Apart from the effectiveness of TGF-ί1, its dosing scheme should be examined, as low doses of intra-articular injection of adenoviruses containing TGF-ί cDNA have no therapeutic or harmful effect on arthritic joints, whereas high levels of TGF-ί increase production of nitric oxide (indicating inflammation), muscle edema and reduced movement of the joint [49].
Bone morphogenic proteins
BMP-2 and BMP-7 are the most commonly used BMPs in gene delivery studies for cartilage, being part of the TGF superfamily. In one of the first studies, periosteal mesenchymal stem cells were transfected with BMP-7 cDNA [46]. Then the modified cells were embedded in polyglycolic acid (PGA) scaffolds and placed in full thickness defects within the rabbit knee. The finding of the study was that transduced cells placed in PGA scaffolds did significantly better than non-transduced cells placed in scaffolds, in terms of forming a larger amount of hyaline cartilage and of performing a quicker restoration of the subchondral bone (as early as six weeks) [56]. However, BMPs must be used with caution for cartilage regeneration as they are well known stimulators of ossification.
Insulin-like growth factor-1
IGF-1 is recognized as a growth factor that induces cartilage proteoglycan synthesis and collagen matrix production and has also been studied in animal models with positive results. Recombinant IGF-1 has a very short half life and this has led researchers to seek a gene delivery system that can modify cells to synthesize their own IGF-1 for extended periods of time. It has been found that modified pristine equine chondrocytes, bone marrow derived chondroprogenitor cells, and synovial cells with IGF-1 cDNA are capable of maintaining therapeutic levels of IGF-1 expression for up to 28 days in in vitro monolayer cultures [36]. In the same study, transfected chondrocytes were found to maintain their morphology and secrete significantly greater amounts of proteoglycans and collagen II. However, a following study, carried out by different investigators, found that articular chondrocytes transfected with IGF-1 did not show a significant increase in collagen II expression, although histologically they scored higher than untreated controls [57]. This discrepancy can be attributed to differences in setup between the two experiments, concerning the vectors used and whether they took place in vivo or in vitro. A next study examined rat articular chondrocytes that were transfected ex vivo with IGF-1 cDNA via adenoviral vectors. These cells were then implanted in partial thickness articular cartilage defects. These chondrocytes preserved their chondrocytic morphology and formed a structure resembling hyaline cartilage after 8 weeks. Similar results were obtained with the use of rabbit articular chondrocytes that underwent genetic modification [58]. IGF-1 gene seems to be promising in the genetic therapy of osteoarthritis, but the outcome of long term expression of IGF-1 needs to be clarified, especially in terms of mechanical properties of the new construct.
Other anabolic gene candidates
TGF-ί1, IGF-1 and BMPs are the most common targets in experiments involving gene delivery for cartilage regeneration. However, they are not the only ones, as some other biological molecules such as transcription factors, intracellular signaling molecules and growth factors are also under investigation and seem to be promising when they are up-regulated. Among them there are Sox-9, Sox-5 and Sox-6, the first set of transcription factors to be identified as essential and sufficient for cartilage formation [59,60]. Fibroblast growth factor (FGF-3) receptor signaling is sufficient to induce chondrogenic differentiation, too, as it has already been shown [61]. Another important group of intracellular regulators of chondrocytic differentiation is formed by signal transduction molecules (SMADs). These molecules function intracellularly, so they cannot be delivered to cells in a soluble form, which means that gene therapy is perhaps the only effective technique through which these molecules can be utilized for cartilage regeneration [44]. Other secreted proteins, such as indian hedgehog (IHH) or sonic hedgehog (SHH), play key roles in regulating chondrocyte hypertrophy [62], and may also prove to be beneficial for modulating the chondrocytic phenotype of grafted cells. Alternatively, delivery and expression of cDNAs encoding specific extracellular matrix (ECM) components such as collagen type II, tenascin, or cartilage oligomeric matrix protein (COMP), may also be used to support production and maintenance of the proper hyaline cartilage matrix [24].
Anti-inflammatory agents, inhibitors of angiogenesis and inhibitors of apoptosis
Enhancement of the anabolic activity of cells in order to synthesize extracellular matrix molecules or to imitate the differentiation observed in embryonic development is the main process in cartilage regeneration. However, as inflammation is a major part of the pathology of damaged cartilage, tissue engineering of damaged cartilage cannot ignore the presence of inflammatory agents. Anti-inflammatory agents have been mainly investigated in animal models where arthritis is induced using collagen or other stimulatory molecules. IL-1 receptor antagonist, soluble TNF-alpha receptors, anti-inflammatory cytokines such as IL-10, IL-4 and IL-13 have been examined and all of them have been successful at decreasing inflammatory response in animal models [47,63-65]. Other effective strategies involve using tissue inhibitors of matrix metalloproteinases (TIMPs), as matrix metalloproteinases (MMPs) are secreted by synovial cells following induction of arthritis and cause severe destruction of the cartilage ECM. In addition, MMPs are also responsible for the cellular invasion of the joint by inflammatory cell. As the action of MMPs is distractive to the joint, tissue engineering strategies have been applied in order to modify arthritic synovial cells to over-express TIMPs. The studies showed a 25% and 13% decrease respectively in the number of invading cells, when synovial cells transfected with TIMP-1 and TIMP-3 were administered in the joint. Apart from reduced levels of active MMPs, significantly reduced cell proliferation was also observed.
A newer approach to the treatment of osteoarthritis through the application of anti-inflammatory agents is the use of pro-opiomelanocortin (POMC). POMC is a precursor of various neuropeptides. POMC-derived neuropeptides are potent inflammation inhibitors and immunosuppressants. What was found is that intra-articular injection of adenoviral vectors expressing POMC significantly suppressed the progression and severity of OA, and reduced inflammatory responses and angiogenesis [66]. In osteoarthritis, angiogenesis, which occurs in the osteochondral junction and synovium, may accelerate inflammation and contribute to the severity of the disease. The inhibition of angiogenesis was assessed in a study testing thrombospondin-1 (TSP-1), an angiogenesis inhibitor, in a rat model of osteoarthritis. This study concluded that in vivo adenovirus-mediated TSP-1 gene transfer significantly reduced microvessel density, inflammation, and suppressed the progression of osteoarthritis [67]. Relevant were also the results of other studies, which assessed the consequences of VEGF blocking. This blocking led to decreased apoptosis of chondrocytes, resulting in cartilage regeneration and a better outcome [68-69].
Inhibitors of apoptosis or senescence, such as Bcl-2, Bcl-XL, hTERT, i(NOS), HSP 70 and others (Table 1), may also be beneficially employed in order to maintain cell populations at the injury site, which are capable of favorable repair responses [70-72].
Table 1. Principles, mechanisms of action and gene candidates for articular cartilage repair.
Mechanism of action |
Target gene |
Inhibition of catabolic pathways |
Inhibition of matrix- degrading enzymes |
TIMP |
Inhibition of proinflammatory cytokines |
IL-1RA |
Chondroprotective cytokines |
IL-4, IL-10 |
Stimulation of anabolic pathways |
Growth factors |
IGF-I, FGF-2,
BMPs, TGF-β |
Chondrogenic transcription factors |
SOX5, SOX6,
SOX9 |
Cytoprotection/proliferation |
Growth factors |
IGF-I, FGF-2 |
Inhibition of apoptosis |
bcl-2, HSP70 |
Catalytic component of human telomerase |
human
telomerase |
Combinatorial approaches |
Inhibition of catabolic pathways plus activation of anabolic pathways |
IGF-I/IL-1Ra;
IGF-I/IL-4 |
Activation of anabolic plus proliferative pathways |
FGF-2/SOX9;
FGF-2/IGF-I |
The role of inhibition of Dkk-1 seems to be interesting too. In a rat experimental model, anterior cruciate ligament transaction and collagenase-induced knee OA was treated with end-capped phosphorothioate Dkk-1 antisense oligonucleotide (Dkk-1AS) which was found to provide therapeutic potential for alleviating cartilage destruction and subchondral bone damage in OA knee joints [73].
To conclude, as both anti-inflammatory agents and anabolic factors play their roles, which are discrete, a combination of synovial cells overexpressing anti-inflammatory agents (e.g. IL-1Ra) and of chondrocytes or stem cells over-expressing one or more anabolic factors (e.g. IGF-1) would probably be the most effective, as it would induce cartilage regeneration and inhibit destruction observed in articular diseases [69,74-77].
Candidate gene delivery vectors
Viral
vectors
Viruses form the one of the two main classes of gene delivery vectors, the other one being non-viral agents, such as polymers and liposomes. Since the idea of gene therapy moved from theory to practice, an intense debate has aroused around the use of viral versus non-viral vectors. Most documented studies involving gene delivery for cartilage regeneration seem to prefer by far viral rather than non-viral vectors. The reason for this preference is simply the superior ability of viruses to transfect cells, as they can attain transfection efficiencies of around 8090%, whereas non-viral vectors can at most transfect 4050% of the cell population. On the other hand, and even though this obstacle exists, non-viral vectors have some advantages that in no way can be ignored, such as ease of synthesis, low immunogenicity and unrestricted plasmid size. As it can be easily understood, these are the main limitations in the utilisation of viral vectors for gene therapy, the most important of them being immunogenicity. Viral vectors induce an inflammatory response, which can lead to various side effects starting from mild oedema and ending to multi-system organ failure. By the time the virus gets recognized by the hosts immune system, repeated administration of the viral vector becomes more and more difficult, as the hosts immune response gets enhanced. The changes that occur to cell surface markers of transfected cells, decrease the possibility of targeting this cell population for a second round of gene delivery. Apart from this obstacle, transfected cells synthesize not only proteins encoded in the genes that were introduced, but also viral markers, which means that the duration of protein synthesis is limited and comes to an end when the inflammatory cells identify these cells and eliminate them. This issue has been partly addressed through the modification of virus genomes in order to minimize the amount of remaining viral genes. Some of these modifications have led to extended expression of transgenes carried by the viruses for as long as 84 days [78].
Even though serious progress has been achieved, direct injection of viral vectors is still considered to be harmful, as dose dependent inflammatory response in joints of various animals has been shown by independent studies [79]. And the problems caused by the intra-articular injection of viruses do not end here, as viruses seem to spread to other organs, even though this dispersion has not been confirmed in larger animals such as rabbits and rhesus monkeys [80].
In order to eliminate most of the problems related to viral gene delivery and, also, retain its benefits, ex vivo gene delivery has been developed. In this technique gene delivery takes place in vitro, where extracted cells get transfected after being expanded in culture. The final stage of this technique, also called cell mediated gene transfer, is the reintroduction of these cells into the body, where they go on producing high quantities of the protein of interest. This technique is ideal as it avoids bringing the virus in direct contact with the body, even though it is not sure that viral genomes are not left behind in ex vivo transfected cells. What is already proved is that there is significant increase in the duration of protein expression with ex vivo gene delivery when compared to direct injection of viral vectors [80]. For example, IL-1RA expression was maintained for up to 6 weeks at rabbit synovial cells transfected ex vivo with a retrovirus [47]. An even more promising finding of a more recent work was that, when not recognized by the immune system, transgene cells lining the synovial cavity go on transcribing the transfected cDNA at relevant levels for up to 6 months [45]. But it is not only the synovial cells that can be transfected through this procedure. Modified articular chondrocytes as well as chondroprogenitor cells that secrete higher amounts of proteins can be directly injected within joints, where they are shown to preferentially adhere to damaged cartilage [57].
Non-viral vectors
The two non-viral gene delivery methods that have so far been utilized for gene delivery for cartilage regeneration are FuGene 6 [81] and modified cationic liposomes [82]. FuGene 6 has been successfully used to transfect a variety of cell lines and is a non-liposomal lipid formulation. The mean transfection efficiency, when FuGene6 was used, was around 35%. Among the transfected chondrocytes, 60% of the cells expressed the transgene IGF-1 for as long as 6 weeks, sustaining therapeutic levels for 32 days and showing a peak at day 5
.
Transfection using the poly-L-lysine lipids [82] involved a multi-step process where transfection was optimized by introducing cells in a detergent (lysolethicin) to permeabilize the cell membrane. Furthermore, transferrin was covalently attached to the polycationic backbone to promote electrostatic interaction with DNA. Transfection efficiency by this method was reported at 71% in vitro. The transgene expression was maintained up to 13 days after transfection and no immune response was observed in vivo in animal models. Future studies will undoubtedly incorporate novel non-viral gene delivery vectors, including polymeric vectors that are currently being developed.
All the above are summarized in Diagram 1.
Diagram 1
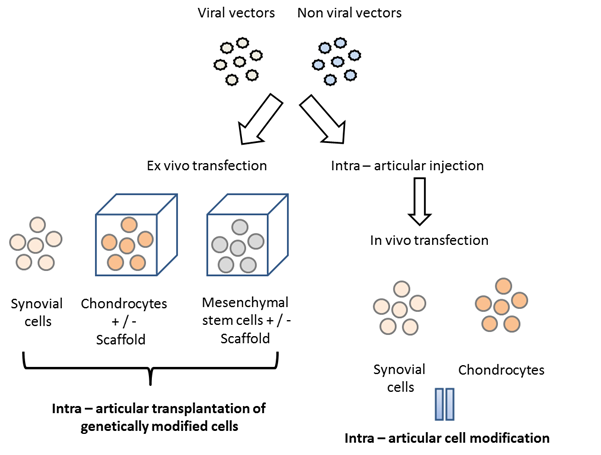
The future of gene therapy in cartilage repair
The construction of a repair tissue that is comparable to the native cartilage tissue in terms of quality and stability is far from being achieved with the use of current cartilage repair approaches.
To overcome various obstacles toward successful repair, including matrix degradation, differentiation or integration insufficiencies, or loss of the transplanted cells and tissues, efficient delivery of chondrogenic, anti-inflammatory, and anti-oxidative factors seems to be crucial (Table 1). Most of these factors are recombinant proteins, possessing a short half life, which means that repeated local administration is demanded to achieve the desired result; a fact leading to delivery problems. The limitations of the current treatments for damaged articular cartilage could be overcome through the adoption of gene transfer techniques. Various approaches have been shown to be suited for efficient transfer of exogenous cDNAs to cartilage defects in vivo, and for achieving sustained expression of the corresponding gene products. Initial efficacy studies indicate that gene-transfer techniques are potent tools that can stimulate a relevant biological response in vivo. To date most approaches delivered a strong anabolic transgene aiming to achieve formation of a hyaline-like cartilage repair tissue in vivo, but with limited long-term success thus far. As the boundaries of current approaches become more clear, it is understood that the future challenge is to determine which combination of transgenes will be most suitable for which aspects of repair, and how best to deliver and express them. The use of more refined vector systems seems to be crucial. Current gene transfer studies to cartilage repair have used vector systems with strong, viral-based promoters, which enabled very high levels of expression, thus facilitating study of the biological effects that may be achieved with a particular gene and gene delivery method. However, it is likely that the stimulation of synthesis of new cartilage resembling the damaged hyaline cartilage and the long-term maintenance of this tissue will require the use of more sophisticated vector systems capable of coordinate control of expression. As many gene products proposed for use can have detrimental side effects if overexpressed in non-target organs such as the heart, lung or kidney, the characterization of the duration of expression in vivo and the distribution of vector and/or genetically modified cells following delivery, will be critical. Toward this end, there are several types of cartilage-specific regulatory elements that have been characterized and that might be incorporated into gene delivery systems, such as promoters for the cartilage-derived retinoic acid-sensitive protein (CD-RAP), the procollagen type II a1 (COL2A1), or the aggrecan gene [83-91].
As cartilage injuries are not life-threatening, the safety of gene transfer approaches for repair is of particular importance. So the transition of this technology to clinical use is strongly dependent on the development of safe and efficient vectors, transgenes and delivery systems.
Reference:
1. Visna P, Pasa L, Cizmar I, et al. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques a randomized controlled study. Acta Chir Belg 2004;104:709714.
2. Bachmann G, Basad E, Lommel D, et al. MRI in the follow-up of matrix-supported autologous chondrocyte transplantation (MACI) and microfracture. Radiologe 2004;44:773782.
3. Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther 1998;28(4):192202.
4. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 2002;10(6):432463.
5. Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum 1998;41(8):13311342.
6. Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopedics 1997;20(6):525538.
7. Minas T. The role of cartilage repair techniques, including chondrocyte transplantation, in focal chondral knee damage. Instr Course Lect 1999:48629643.
8. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 2001;(391 Suppl):S362369.
9. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 2002;15(3):170176.
10. Bouwmeester SJ, Beckers JM, Kuijer R, et al. Long-term results of rib perichondrial grafts for repair of cartilage defects in the human knee. Int Orthop 1997;21(5):313317.
11. Caplan AI, Elyaderani M, Mochizuki Y, et al. Principles of cartilage repair and regeneration. Clin Orthop 1997;342:254269.
12. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 2003;85-A:22532.
13. Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331(14):889895.
14. Peterson L, Brittberg M, Kiviranta I, et al. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 2002;30(1):212.
15. Peterson L, Minas T, Brittberg M, et al. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 2003:21724.
16. Pascher A, Steinert AF, Palmer GD, et al. Enhanced repair of the anterior cruciate ligament by in situ gene transfer: evaluation in an in vitro model. Mol Ther 2004;10(2):327336.
17.Kuo CK, Li WJ, Mauck RL, et al. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol 2006;18(1):6473.
18. Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther 2003;5(5): 235238.
19. Hidaka C, Goodrich LR, Chen CT, et al. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res 2003;21(4):573583.
20. Nixon AJ, Fortier LA, Williams J, et al. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res 1999;17(4):475487.
21. Nixon AJ, Saxer RA, Brower-Toland BD. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-I autocrine/paracrine response in chondrocytes. J Orthop Res 2001;19(1):2632.
22. Shuler FD, Georgescu HI, Niyibizi C, et al. Increased matrix synthesis following adenoviral transfer of a transforming growth factor beta1 gene into articular chondrocytes. J Orthop Res 2000;18(4):585592.
23. Smith P, Shuler FD, Georgescu HI, et al. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum 2000;43(5):11561164.
24. Dharmavaram RM, Liu G, Tuan RS, et al. Stable transfection of human fetal chondrocytes with a type II procollagen minigene: expression of the mutant protein and alterations in the structure of the extracellular matrix in vitro. Arthritis Rheum 1999;42(7):14331442.
25. Li Y, Tew SR, Russell AM, et al. Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng 2004;10(34):575584.
26. Tew SR, Li Y, Pothacharoen P, et al. Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage 2005;13(1):8089.
27. Enomoto H, Enomoto-Iwamoto M, Iwamoto M, et al. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem 2000;275(12):86958702.
28. Iwamoto M, Kitagaki J, Tamamura Y, et al. Runx2 expression and action in chondrocytes are regulated by retinoid signaling and parathyroid hormone-related peptide (PTHrP). Osteoarthritis Cartilage 2003;11(1):615.
29. Kolettas E, Muir HI, Barrett JC, et al Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology (Oxford) 2001;40:11461156.
30. Hardingham T, Tew S, Murdoch A. Tissue engineering: chondrocytes and cartilage. Arthritis Res 2002;4(Suppl 3):S63S68.
31. Young AA, Smith MM, Smith SM, et al. Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res Ther 2005;7:R852 R861.
32. Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Jt Surg Am 1994;76:579592.
33. Solchaga LA, Goldberg VM, Caplan AI. Cartilage regeneration using principles of tissue engineering. Clin Orthop Relat Res 2001:S161S170.
34. Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. Curr Opin Biotechnol 2004;15:406410.
35. Saxer RA, Bent SJ, Brower-Toland BD, et al. Gene mediated insulin-like growth factor-I delivery to the synovium. J Orthop Res 2001;19:759767.
36. Nixon AJ, Brower-Toland BD, Bent SJ, et al. Insulin-like growth factor-I Gene Therapy applications for cartilage repair. Clin Orthop Relat Res 2000:S201S213.
37. Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Jt Surg Am 1997;79:14521463.
38. Gelse K, von der Mark K, Aigner T, et al. Articular cartilage repair by Gene Therapy using growth factor-producing mesenchymal cells. Arthritis Rheum 2003;48:430441.
39. Grande DA, Mason J, Light E, et al. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am 2003:2111116.
40. Katayama R, Wakitani S, Tsumaki N, et al. Repair of articular cartilage defects in rabbits using CDMP1 gene-transfected autologous mesenchymal cells derived from bone marrow. Rheumatology (Oxford) 2004;43(8):980985.
41. Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003;102:38373844.
42. Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:1120.
43. Evans CH, Ghivizzani SC, Kang R, et al. Gene Therapy for rheumatic diseases. Arthritis Rheum 1999;42:116.
44. Trippel SB, Ghivizzani SC, Nixon AJ. Gene-based approaches for the repair of articular cartilage. Gene Ther 2004;11:351359.
45. Gouze E, Pawliuk R, Pilapil C, et al. In vivo gene delivery to synovium by lentiviral vectors. Molec Ther 2002;5:397404.
46. Makarov SS, Olsen JC, Johnston WN, et al. Suppression of experimental arthritis by gene transfer of interleukin 1 receptor antagonist cDNA. Proc Natl Acad Sci U S A 1996;93:402406.
47. Evans CH, Ghivizzani SC, Herndon JH, et al. Clinical trials in the Gene Therapy of arthritis. Clin Orthop Relat Res 2000:S300S307.
48. Bakker AC, van de Loo FA, van Beuningen HM, et al. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthr Cartil 2001;9:128136.
49. Mi Z, Ghivizzani SC, Lechman E, Glorioso JC, et al. Adverse effects of adenovirus-mediated gene transfer of human transforming growth factor beta 1 into rabbit knees. Arthritis Res Ther 2003;5:R132R139.
50. Tuli R, Tuli S, Nandi S, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem 2003;278:4122741236.
51. Shuler FD, Georgescu HI, Niyibizi C, et al. Increased matrix synthesis following adenoviral transfer of a transforming growth factor beta1 gene into articular chondrocytes. J Orthop Res 2000;18:585592.
52. Kofron MD, Laurencin CT. Orthopaedic applications of Gene Therapy. Curr Gene Ther 2005;5:37 61.
53. Alvarez J, Sohn P, Zeng X, et al. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development 2002;129:19131924.
54. Pepper MS, Montesano R, Vassalli JD, et al. Chondrocytes inhibit endothelial sprout formation in vitro: evidence for involvement of a transforming growth factor-beta. J Cell Physiol 1991;146:170179.
55. Lee JY, Hall R, Pelinkovic D, et al. New use of a three-dimensional pellet culture system for human
intervertebral disc cells: initial characterization and potential use for tissue engineering. Spine 2001;26:23162322.
56. Grande DA, Mason J, Light E, et al. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Jt Surg Am 2003;85-A(Suppl 2):111116.
57. Madry H, Kaul G, Cucchiarini M, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther 2005;12:11711179.
58.Smith P, Shuler FD, Georgescu HI, et al. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum 2000;43:11561164.
59. Semba I, Nonaka K, Takahashi I, et al. Positionally-dependent chondrogenesis induced by BMP4 is co-regulated by Sox9 and Msx2. Dev Dyn 2000;217:401414.
60. Ikeda T, Kamekura S, Mabuchi A, et al. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum 2004;50:35613573.
61. Hoffmann A, Czichos S, Kaps C, et al. The T-box transcription factor Brachyury mediates cartilage development in mesenchymal stem cell line C3H10T1/2. J Cell Sci 2002;115:769781.
62. Vortkamp A. Interaction of growth factors regulating chondrocyte differentiation in the developing embryo. Osteoarthritis Cartilage 2001:AS109117.
63. Apparailly F, Verwaerde C, Jacquet C, et al. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol 1998;160:52135220.
64. Bessis N, Honiger J, Damotte D, et al. Encapsulation in hollow fibres of xenogeneic cells engineered to secrete IL-4 or IL-13 ameliorates murine collagen- induced arthritis (CIA). Clin Exp Immunol 1999;117:376382.
65. Bakker AC, Joosten LA, Arntz OJ, et al. Prevention of murine collagen-induced arthritis in the knee and ipsilateral paw by local expression of human interleukin-1 receptor antagonist protein in the knee. Arthritis Rheum 1997;40:893900.
66. Shen PC, Shiau AL, Jou IM, et al. Inhibition of cartilage damage by pro-opiomelanocortin prohormone overexpression in a rat model of osteoarthritis. Exp Biol Med (Maywood) 2011 Mar 1;236(3):334-340.
67. Hsieh JL, Shen PC, Shiau AL, et al. Intra-articular gene transfer of thrombospondin-1 suppresses the disease progression of experimental osteoarthritis. J Orthop Res 2010 Oct 28(10):1300-6
68. Matsumoto T, Cooper GM, Gharaibeh B, et al. Blocking VEGF as a potential approach to improve cartilage healing after osteoarthritis. J Musculoskelet Neuronal Interact 2008; 8(4):316-317.
69. Matsumoto T, Cooper GM, Gharaibeh B, et al. Cartilage repair in a rat model of osteoarthritis through intra-articular transplantation of muscle-derived stem cells expressing Bone Morphogenetic Protein 4 and soluble Flt-1. Arthritis Rheum 2009;60(5):13901405
70. D'Lima DD, Hashimoto S, Chen PC, et al. Impact of mechanical trauma on matrix and cells. Clin Orthop Relat Res 2001;(391 Suppl):S9099.
71. D'Lima DD, Hashimoto S, Chen PC, et al. Cartilage injury induces chondrocyte apoptosis. J Bone Joint Surg Am 2001;83-A(Pt 1):1921.
72. Grossin L, Cournil-Henrionnet C, Pinzano A, et al. Gene transfer with HSP 70 in rat chondrocytes confers cytoprotection in vitro and during experimental osteoarthritis. FASEB J. 20, 6575 (2006).
73. Weng LH, Wang CJ, Ko JY, et al. Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees. Arthritis Rheum 2010;62(5):1393-1402.
74. Haupt JL, Frisbie DD, McIlwraith CW, et al. Dual transduction of insulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J Orthop Res 2005;23(1):118126.
75. Nixon AJ, Haupt JL, Frisbie DD, et al. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther 2005;12(2):177186.
76. Wang HJ, Yu CI, Kishi H, et al. Suppression of experimental osteoarthritis by adenovirus- mediated double gene transfer. Chin Med J 2006; 119 (16):1365-1373.
77. Biao C, Jun Q, Hui W, et al.Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med 2010;42 (10): 684-695.
78. Verma IM, Somia N. Gene Therapy promises, problems and prospects. Nature 1997;389:239242.
79. Lubberts E, Joosten LA, Chabaud M, et al. IL-4 Gene Therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest 2000;105:16971710.
80. Gelse K, Jiang QJ, Aigner T, et al. Fibroblast mediated delivery of growth factor complementary DNA into mouse joints induces chondrogenesis but avoids the disadvantages of direct viral gene transfer. Arthritis Rheum 2001;44:19431953.
81. Madry H, Kaul G, Cucchiarini M, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther 2005;12:11711179.
82. Goomer RS, Maris TM, Gelberman R, et al. Nonviral in vivo Gene Therapy for tissue engineering of articular cartilage and tendon repair. Clin Orthop Relat Res 2000:S189S200.
83. Lefebvre V, Mukhopadhyay K, Zhou G, et al. A 47-bp sequence of the first intron of the mouse pro alpha 1(II) collagen gene is sufficient to direct chondrocyte Expression. Ann N Y Acad Sci 1996:785284287.
84. Naski MC, Colvin JS, Coffin JD, et al. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development 1998;125(24):49774988.
85. Naski MC, Ornitz DM. FGF signaling in skeletal development. Front Biosci 1998:3d781794.
86. Valhmu WB, Palmer GD, Dobson J, et al. Regulatory activities of the 5'- and 3'-untranslated regions and promoter of the human aggrecan gene. J Biol Chem 1998;273(11):61966202.
87. Zhou G, Lefebvre V, Zhang Z, et al. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J Biol Chem 1998;273(24):1498914997.
88. Tsumaki N, Tanaka K, Arikawa-Hirasawa E, et al. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol 1999;144 (1):161173.
89. Xie WF, Zhang X, Sakano S, et al. Trans-activation of the mouse cartilage-derived retinoic acid sensitive protein gene by Sox9. J Bone Miner Res 1999;14(5):757763.
90. Lian JB, Stein GS, Stein JL, et al. Marrow transplantation and targeted gene therapy to the skeleton. Clin Orthop Relat Res 2000;(379 Suppl):S146155.
91. Smith N, Dong Y, Lian JB, et al. Overlapping expression of Runx1 (Cbfa2) and Runx2 (Cbfa1) transcription factors supports cooperative induction of skeletal development. J Cell Physiol 2005;203(1):133143.
|



