Address for Correspondence
Nicholas Ali
125 University Private, Rm. 319, University of Ottawa, K1N 6N5, Canada,
Phone:1-613-884-755
Email addresses:NA: nali065@uottawa.ca
Abstract
Substantial research to better understand anterior cruciate ligament (ACL) injury during non-contact sporting events is yet to yield consensus on risk factors. Non-contact ACL injury likely occurs when many extreme conditions and risks factors happen at the same time. Limitation of current study methodologies is their inability to simultaneously capture many of these contributing factors to injury. In spite of considerable research precise knowledge about how and why the ACL gets injured is still unknown. This paper aims at elucidating the key challenges that confound our understanding in establishing the main determinants of ACL injury during non-contact sporting events. This work also teases out gaps in knowledge to steer future research and presents a possible approach to better understand ACL injury causations. This review unveils that new research modalities or coupled research methods are required to better understand how and why the ACL gets injured. Only by achieving a better understanding of these contributing factors, one will be able to develop robust prevention strategies and exercise regimens to mitigate ACL injuries.
J.Orthopaedics 2011;8(3)e12
Keywords:
Anterior cruciate ligament (ACL), non-contact ACL injury, risk factors, soft tissues, hip and ankle
Introduction:
Even though the anterior cruciate ligament (ACL) can be injured during an impact event such as a car crash, studies have demonstrated that 70 to 80% of ACL injuries occur during non-contact sporting events, which involve sudden deceleration, a change in direction, or landing on one leg as is common in soccer, basketball, handball, and volleyball [1, 2]. Thus, there are many ways that the ACL can be overloaded during sporting activity. Increased participation in athletic activities, especially among females, has resulted in an increase of the number of ACL injuries. Approximately 100,000 to 175,000 ACL-related surgeries are conducted in the United States each year [3, 4], with associated costs exceeding $2 billion [5]. Among athletes, female’s ACL injuries are 2-8 times more frequent than in males [6]. Complete ACL rupture may lead to significant posttraumatic laxity, functional knee instability, and increased likelihood of osteoarthritis [7]. The most common treatment for a ruptured ACL is surgery.
During the 1990s, there was a paradigm shift in research focus on the ACL, from the development of more effective diagnostics and treatments to greater emphasis on identifying the mechanisms and risk factors of non-contact ACL injuries [3]. This shift was driven by the realization that only if the causal relations between applied forces and the resultant injury are understood, then appropriate programs of intervention and prevention can be designed and implemented [8]. This focus persists, but research has been somehow patchy and somewhat stagnant. Nonetheless, the prevention of sport related non-contact ACL injuries today relies largely on the ability to screen and then modify identified risk factors through training. Neuromuscular activation and muscle strength are among the risk factors that can be modified through training.
Being able to better identify and categorize risk factors (e.g. high, medium and low risk), as contributors to non-contact ACL injuries may help provide pre-screening tools, tailored preventative and exercise regimens, and aid in the design of better intervention devices. However, knowing that many risk factors and extreme conditions act in concert to cause ACL injury, poses a very complex task for any research project.
The goal of this work is to provide a comprehensive assessment of the challenges that hinder our ability to precisely identify risk factors of non-contact ACL injury. This is a crucial task since the literature is replete with discrepancies, a lack of consensus, coherence, and confusion. Understanding these challenges is a necessary first step towards designing studies to better understand ACL injury mechanisms and developing injury prevention programs. This article highlights the grave needs and promising opportunities to steer future research direction in ACL injury biomechanics. It focuses on two important questions: 1) What are the challenges to clearly identifying the determinants of ACL injury during non-contact sporting events; and 2) What are the gaps in knowledge as a result of these challenges?
Material and Methods :
This article reviewed the relevant literature on ACL injury mechanisms in the PubMed electronic database using MEDLINE (1966 to 2007), Proquest (1987 to Sept. 2007), and, Applied and Complementary Medicine Database (AMED) on Ovid (1985 to Sept. 2007). Keywords used in our search included “anterior cruciate ligament”, “risk factors”, and “non-contact injuries”. A total of 1,756 articles were identified and reviewed. The most relevant full text English articles pertaining to ACL non-contact injuries, risk factors for ACL injuries, and study approaches to understand ACL mechanics were analyzed. Studies that captured the association of ACL and non-contact injuries, and ACL with a specific study approach were also included. Our search was supplemented by reviewing the bibliographies of retrieved articles, as well as, hand searching scholarly journals outside of the bio-fields related to this topic.
Challenges identifying risk factors of non-contact ACL injury
High incidences of ACL injury, frequent requirements for ACL reconstruction and limited understanding of ligament mechanics have engendered considerable interest in quantifying ACL loading, especially during movement. Despite substantial research to understand knee mechanics, precise evidence and explanation about how and why ACL injuries occur is still largely unavailable. Biomechanics, video analysis and related study approaches have elucidated, to some extent, how ACL injuries occur, but they are limited because they provide estimates, rather than precise measurements of knee, and more specifically ACL kinematics at the time of injury. More importantly, little is known about ligament and muscle loading and response during ACL injuries. This may be attributable to the innate difficulties associated with measuring ligament and muscle forces in-vivo. It is understood, based on this review, that most of the research on ACL injuries has focused on the effects of knee mechanics before and after ACL failure, or before and after ACL reconstruction, but not during the course of ACL injury. Furthermore, the vast majority of studies on ACL injuries are in the context of reconstruction and surgical treatment, but not on the very important aspect of precisely how and why the ACL gets injured.
The success of any screening method relies on a precise understanding of the relationship between knee motion, ACL load, and the subsequent risk of injury. Until accurate descriptions of these relationships are available, the potential exists for all screening methods to exclude incorrectly “at risk” people from any ensuing intervention process [9]. For athletes who are at higher risk, a complete understanding of injury causation needs to address the multi-factorial nature of sport injuries [10]. The following section highlights some of the major impediments to furthering our understanding of the determinants of non-contact ACL injury.
Shortcomings and general inherent obstacles of studying non-contact ACL injury
For ethical reasons, in-vivo measurements of ACL loading to failure in human or animal subjects cannot be undertaken. As well, relationships between internal muscle forces, external loading, and ACL loads are mostly unknown due to the difficulties of measuring ligament and muscle forces in-vivo. Moreover, maximum kinematic changes of knee, for example, anterior tibial translation (ATT) may not necessarily correspond to maximum force in the ACL due to the concurrent interaction of multiple tissues and the interplay between multiple risk factors implicated to cause ACL injuries. These concurrent interactions are very difficult to capture with most current study approaches. This is complicated further by the structural complexity of the knee, the multiple structures that contribute to similar function, the structural complexity of the ACL and its non-linear stress-strain relation, and the interactions between these structures.
The difficulties in obtaining material property data for hard and soft human tissues also create some limitations, especially for computational modeling studies. Other challenges include high cost of experimental studies, as well as, the difficult and sometimes impossible task of reproducing certain natural, pathological or degenerative situations in-vitro. In addition, there can be significant inter- and intra- subject variability during experimental testing Fragmentation and discrepancies in the literature may be a reflection of the limitations and differences in current ACL injury study approaches. For example, some studies have shown that knee compression increases ACL strain [11, 12]. However, other studies have demonstrated that the ACL is protected during compressive loading at the tibio-femoral (TF) joint since the load presses the articular surfaces together and limit anterior tibial translation (ATT), thereby limiting . strain at the ACL [13]. The following subsections elucidate specific areas where inherent challenges and difficulties in studying non-contact ACL injury persist.
Muscle Not Included in Problem Definition
A study that does not include muscles cannot adequately predict risk factors of ACL injury. Omitting muscles leads to inaccuracies since the forces transmitted to the ligaments and bones are dominated by muscle forces [14]. It appears that ligaments can only play a limited role in maintaining the integrity and stability of the knee joint. In fact, it is very likely that ligament rupture occurs only when muscle action fails to protect them. The large difference in ACL injuries between men and women [1] may be an indicator that muscle incorporation is important for any study investigating soft tissue injury biomechanics. Muscle recruitment strategies may be one indicator of why females suffer more ACL injuries than men [15, 16], and strongly points to the importance of musculo-tendon contributions in maintaining knee joint stability and integrity. The two paragraphs to follow highlight some discrepancies with regards to the role of muscles in loading the ACL.
In-vivo studies [17] have concluded that ACL strain increases if there is an increase in quadriceps activity relative to hamstring activity. McConkey and colleagues [18] are probably the first to describe eccentric quadriceps contraction as the intrinsic force responsible for ACL injury. Numerous in-vitro studies using defect free cadavers have also focused on the effects of increased quadriceps forces on ACL strain [19, 20] and concluded that increased quadriceps forces induce ATT, and consequently increase the force in the ACL over a specific range of knee flexion. One in-vivo study with healthy men and women (using 11 subjects) aged 21-42 years and with normal ACL [21] showed similar results. However, one in-vitro study using six pairs of human cadaveric knees [22] found that the quadriceps muscle force protected the ACL from injury. As well, one study demonstrated that under non-physiological loading without ground reaction forces (GRFs), the knee locked in one position, and when static quadriceps loads of 4500 N were applied, ACL injury did occur [23]. The authors suggested quadriceps drawer as an injury mechanism. Hamstring muscle force applies a posterior directed force component and has been reported to strain shield the ACL by reducing ATT at large flexion angles [24]. An early study by Solomonow et al. [25] suggests that strength training of the hamstring muscles can help prevent damage to the intact ACL. On the other hand, a later study by Shelburne et al. [26] demonstrated that the hamstring cannot apply large enough posterior forces to unload the ACL especially at low knee flexion angles- a position where many non-contact ACL injuries occurs. It has also been demonstrated by Simonsen et al. [27] that the ability of the hamstring muscles to reduce the ACL load is marginal.
The results of an in-vivo study by Fleming et al. [28] demonstrated that the gastrocnemius muscle is an antagonist to the ACL. This is in agreement with an analytical study by Pflum et al. [12]. On the other hand, a cadaveric study by Durselen et al. [29], and a theoretical investigation by Shelburne et al. [30], have demonstrated that contraction of the gastrocnemius muscles did not strain the ACL over the entire range of knee flexion.
The discrepancies among these studies highlight the need to determine the contribution of muscle activation and loading on ACL load. Nonetheless, many studies clearly demonstrate that ACL loading is affected by muscle activity and that the muscles must be included in any investigation addressing injury to the ACL. Moreover, considering the limited strength of the ACL, active control of the knee joint may very well depend at any moment on the balance of the resultant muscle forces.
Lack of Studies that Include Ankle and Hip Articulation
The majority of non-contact ACL injury studies do not address the effects of hip and ankle kinematics and kinetics on ACL loads. In addition, the majority of studies in the literature do not account for the effects of whole body movement on ACL loads. The inclusion of the ankle is important in ACL injury studies as it has been shown that the nature of shoe surface interface is an important risk factor in ACL injury [31]. In studies conducted with basketball players over various turf types and soccer players with varying cleat lengths, it was also shown that foot contact with the ground is an important risk factor in ACL injury [32]. As well, at the distal end of the leg the ground reaction forces (GRFs) act through the foot, applying moments and forces to the ankle. Muscular activity across the ankle controls the position of the foot at landing for example, which most likely influence the loading at the ankle [33]. Moreover, muscular activity at the ankle may influence the loads that are transferred through the ankle to the knee [33]. Two studies [12, 34] found that the forces applied by the ground to the foot have a major impact on peak forces at the ACL. Another study found that when the foot was not flat, the GRFs could not be transmitted effectively through the bones to the ground without the actions of muscles [35]. In addition, excessive foot pronation was shown to increase the likelihood of ACL injury [34].
Inclusion of hip articulation is also important to the understanding of how and why the ACL gets injured since hip movement is known to affect the loading on the knee [16]. Because the upper body contains over half of the total body mass, the motion of the upper body should normally affect the loads seen at the knee. The position of the leg at the time of ACL injury displays tibial rotation, apparent knee valgus, foot pronation, and a relatively extended knee and hip [2, 3]. Muscle contraction at the hip can also affect knee loading because the hip transfers the upper body loads to the leg [36]. Moreover, a cadaveric study [37] and an analytical study [38] both demonstrated that if the hip movement is restricted, ATT will result and ACL injury will occur. Many studies [16, 39] also suggested that the knee is one part of the kinetic chain and that the torso, hip, and ankle may also contribute to ACL injury. The importance of including the hip and ankle joint in any ACL injury study is supported by many other studies which are captured in a review article by Hewett et al. [40]. So, one can conclude that non-contact ACL injury is a whole body event that requires the study approach to capture the contributing effects of ankle and hip articulations to ACL injury causation.
Studies Do Not Accurately Capture Articular Surface Geometry
The geometry of the articular surfaces, for example, femoral notch [41] has been implicated as a risk factor for ACL injury. The geometry of the hard tissues aids in knee joint stability. The femoral groove that the patella slide on, the femoral condyles resting on the tibial eminence [42] and the posteriorly sloped tibial plateau [43] may all serve to assist in providing restraints to anterior displacement and rotation and hence assist in preventing ACL injuries. There are many computational studies that overlook the contribution of these factors to ACL injury. Lui et al. [44] found that articular surface geometry, ligament locations, and ligament insertion sites had a pronounced effect on modeling output. Irrespective of muscular activity, Rentrom et al. [45] demonstrated that the ACL is subjected to an inherent increase in strain as the knee extends owing to the geometry of the articular surfaces of the knee. Despite this, many studies have not considered accurate 3D hard and soft tissue geometries and few have looked at the effect of geometry changes on loads seen at the ACL. The simultaneous inclusion of the muscles, hip, knee, and ankle articulations, as well as, accurate 3D tissue geometries appears to be crucial study prerequisites. Without an objective view of these parameters in problem definition, a clear and comprehensive understanding of risk factors of non-contact ACL injury may remain elusive.
Obstacles in current non-contact ACL injury biomechanics study approaches
Experiment, athlete interviews, clinical studies, video analysis, musculoskeletal and computational modeling are all different study approaches that have contributed to understanding ACL mechanics (see Figure 1). A concise overview of most of these approaches along with their strengths and weaknesses are presented by Krosshaug et al. [46]. It seems that one common shortcoming in all these approaches is the inability to fully capture the many factors implicated as contributors to ACL injury. Clinical studies, interviews with athletes, and video analyses provide mostly qualitative data and so are not adequate for obtaining a comprehensive understanding of injury to the ACL. From the current literature, one can glean three main quantitative approaches for better understanding how and why the ACL gets injured. These methods include experimentation, computational modeling, and analytical modeling (highlighted in green in Figure 1). All these methods will be discussed in the context of this review. The number of empirical studies in the literature greatly outnumbered numerical and analytical studies. As well, the number of numerical studies outnumbered analytical studies. Experimentation involves employing essentially two approaches: 1) In-vivo experiments (few); and 2) In-vitro experiments (numerous).
Figure 1- Current approaches employed to better understand non-contact ACL injury
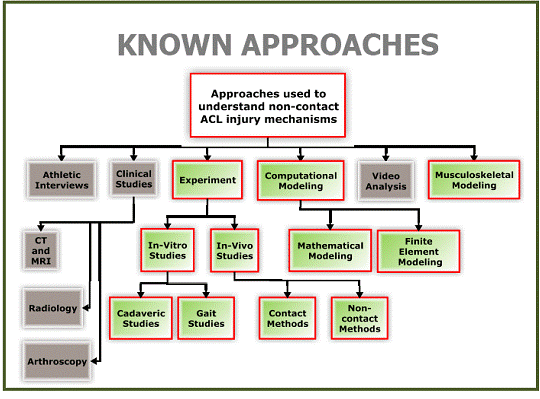
Challenges Facing Field of In-Vivo Experimentation
It can be argued that historically research on the human musculoskeletal system was focused on empirical methods. Berns et al. [47] attributed variations in experimental results to four sources: 1) individual variation in material properties of the hard and soft tissue structures; 2) differences in bone geometry; 3) differences in soft tissue geometry; and 4) experimental errors. The first three continue to challenge experimental investigations aiming to obtain repeatable results and can also be an interfering phenomenon with numerical modeling. However, one other source of variation during experiment not mentioned by Berns et al. [47] is muscle activation and control. One form of in-vivo testing entails the direct implantation of sensors to subjects’ tissues. These are termed contact methods and have the advantage of including many risk factors and forces.
A significant challenge with this form of in-vivo testing is that it is invasive. Moreover, because of the size of the sensor employed and the location of the ACL, in-vivo studies to-date can only measure strain on the anteromedial bundle of the ACL for movements confined to sagittal plane [11]. Measurement is confined to linear displacement at discrete locations and at knee flexion angles approximately greater than 15 degrees [21]. Woo et al. [48] have argued that strain gauge and other similar type transducers require instrumentation and contact with the ligament that alters ligament length, and subsequently, ligament force. Another complicating factor with in-vivo techniques is the fact that specific bands of the ligament are tensioned at different portions of the loading cycle.
Another form of in-vivo testing includes non-contact methods. These methods do not require the implantation of a sensor. Non-contact in-vivo methods include the use of dynamic magnetic resonance imaging (MRI) to capture tissue kinematics at various knee flexion angles. This technique does not provide any details of the forces seen at the ACL during movement.
Other approaches and tools used in the past to measure in-vivo ACL strain include implantable extensometer [21, 49], buckle transducers [50, 51], or optical methods such as Roentgen Stereophotogrammetry [52]. It may be argued that none of the techniques above are truly in-vivo approaches given they alter in one form or the other the way the subject move during testing.
Challenges Facing Field of In-Vitro Experimentation
In-vitro testing is conducted outside of the body typically with human subjects or post mortem human subjects (PMHS)/ cadavers. The vast majority of studies investigating non-contact ACL injury are in-vitro [37, 48, 53-55]. In-vitro testing also has the capability of simulating knee kinematics and muscle loads. Cadaveric studies, however, may not accurately describe ligament function in-vivo since loading applied to the cadavers during experiment is different from that applied by the muscles during activity. The major challenge with in-vitro studies using cadavers is the inability to include muscle activation and the difficulty of obtaining repeatable results. Replicating an isolated ACL injury in-vitro is difficult, and has been achieved with only limited success [37]. This shortcoming may exist because cadaveric knee studies lack the stability and control provided by active knee musculature [56]. It is also difficult to apply large muscle forces seen during normal physical activities, such as walking, to in-vitro experiments using cadavers.
Another prevalent in-vitro study method is gait analysis that employs skin markers. The use of skin markers with gait studies have been shown to induce significant errors in predicting in-vivo ligament behavior [57, 58]. Since some gait studies are conducted in the laboratory setting, there is also some level of uncertainty with respect to the ability of these tools in capturing true human response. For example, since the subject is aware that the camera is focused on them or their feet have to strike a force plate, it can be argued that their body response may be somewhat altered to fit the event. As a result, the data collected may not be a true representation of human motion. This issue has forced many researchers to develop systems to monitor and measure metrics of human movement outside the lab. Despite its shortcomings, in-vitro studies have the capability to provide much freedom to investigate function and behavior of the ACL. It can be argued that gait analysis is the only way available today to determine what motion and forces occur during real activities to cause ACL injury.
Challenges Facing the Computational Modeling Field
There are mainly three approaches to computational modeling in the current literature: mathematical, finite element (FE) modeling, and musculoskeletal rigid body (RB) modeling. A computational model of the knee joint is a graphical representation of the joint anatomy that can be manipulated. Many mathematical modeling investigations of the human knee focus on the intact ACL. With many of these models, if injury is studied, it is done primarily by removing the effect of the ACL in model predictions [59]. Even though mathematical models have aided our understanding of the mechanics of the knee, the gross approximations and assumptions can readily be answered with technology and information available today. Two such technologies are finite element (FE) modeling and RB modeling. Mathematical models have become less popular over recent years perhaps due to: (1) Easy access and lower cost of FE and RB modeling software; and (2) Inherent complexity and nonlinearity in the governing equations related to biomechanics problems, such as the ACL mechanics, and so the challenges entailed in developing and verifying these models. Hefzy et al. [60] provided an excellent review of mathematical models of the human knee joint found in the literature.
Andriachi et al. [61] appeared to be the first to develop a model for the analysis of motion and forces in the knee joint using FE methods. Once verified and validated, a FE model can provide greater capabilities to answer many what if questions over mathematical models. However, mathematical models of the knee joint, even though taking longer to develop compared to FE models, use less computational time and offers greater flexibility. The literature indicates that only a small number of FE models are used to study ACL mechanics, and none has focused on ACL injury mechanisms. Many of these studies do not report data on model verification and validation, thus limiting their use in clinical applications.
Other FE studies investigating ligament mechanics in the literature modeled only the ACL[62-64]. None of these studies investigated non-contact ACL injury. Nonetheless, the challenge with FE modeling of biological tissues include the complexity posed by modeling organic materials geometry, the complexity of modeling material responses under loading, the level of discretization required to capture complex and intricate anatomical geometries, long computational time to converge [65], and dependence on empirical data for validation among others. Increased and affordable computing capacity and more sophisticated (nonlinear and 3D) FE software packages has allowed more realistic modeling and the application of iterative procedures to describe time-dependent mechanical behavior. Nonetheless, this may not be enough to fully address the challenges posed by the complexity of understanding the determinants of ACL injury.
Musculoskeletal RB modeling of the knee is based on research aimed at developing a dynamic
rigid body model for balancing muscle and ligament forces with externally applied forces to produce motion [66-68]. Three main approaches may be used to balance internal muscle forces with externally applied forces for a specific motor task: inverse dynamics; forward dynamics; and optimum control theory. Erdemir et al. [88] provided a thorough review of these three approaches applied to musculoskeletal modeling. The main advantage of musculoskeletal RB modeling is that it enables us to determine forces in the muscles during activities implicated to cause ACL injuries. On the other hand, musculoskeletal models cannot provide details of forces and stresses in the hard and soft tissues, as FE models does. In addition, musculoskeletal modeling can require extremely long computational time to converge to a solution and in some cases requires parallel computing [69].
Shortcomings in biomechanics field
It can be argued that some shortcomings in the biomechanics field pose a major challenge to furthering our understanding of the determinants of non-contact ACL injury. The literature points to some systemic challenges facing the field of biomechanics which are believed to be impeding progress and clouding a collective agreement in the understanding of risk factors of non-contact ACL injury. Biomechanics studies, and more specifically ACL biomechanics studies, are mostly empirical. Empirical testing has been the main approach building the foundation of biomechanics. In fact, there is a significant gap between the advancement of empirical tools and that of analytical and numerical tools, in biomechanics. It has been recognized that there is a lack of consensus in the research community on merit of formulating clinical recommendations based on physical and numerical model results [70]. This may exacerbate itself to much uncertainty in theories used in teaching clinical biomechanics [71].
ACL injury research is a multidisciplinary field, since one need to consult with many disciplines in a single problem. With the cross linking of certain disciplines (such as biomechanical engineering, mechano-biology, bio-infomatics, etc.), problems in non-contact ACL injury research may become more easily solvable. Such cross linking may also bring about new resources, skill sets, approaches, and outlooks to the field of biomechanics. As an unfortunate fact, it seems that there are some shortcomings in the biomechanics field that continue to hinder a clear understanding of the factors that may contribute to the occurrence of ACL injury. The following two sections shed some light into these challenges.
Studies Too Narrowly Focused
Based on this investigation, it is understood that the type and complexity of the research method employed depends on the question(s) posed. Nonetheless, it seems quite reasonable to accept that non-contact ACL injury is multifaceted with numerous risk factors simultaneously at play to cause injury. There are few studies, to our best knowledge, where a tool or methodology is employed that lends itself to simultaneously determining effects of multiple risk factors on ACL injury.
Many studies have pointed out that several intrinsic and extrinsic factors are responsible for non-contact ACL injuries [82]. However, most focused on investigating the effects of a single risk factor by comparing intrinsic or extrinsic differences between males and females, such as Q-angle [83], intercondylar notch width [84], and hormones [85] without considering the combined effects of other contributing factors. In addition, some studies address the effects of only one muscle group on ACL rupture. But, it seems quite unlikely that a single risk factor or only one muscle group will be responsible for ACL injury.
Lack of Standardization
Combining and comparing results from separate studies that use similar research methods can be valuable, but differences in, for example, specimen type and data reporting may prevent drawing solid conclusions about the results. The challenge is these differences may exist due to the lack of guidelines on study method, instrumentation to employ, and procedures for of data analysis. The dearth of standards and specifications in the field of biomechanics is believed to be one reason why dialogue among research groups and comparisons in experimental studies remains challenging, debatable, and sometimes with no solid outcome. A few attempts at standardization that have led to considerable benefits in the research community include the Visible Human Project [86], VAKHUM [87], and the standardized femur [88]. These endeavors have aided in removing inter subject variability, as well as, simplifying cross validation of research results.
Due to the lack of standards and specifications in study approaches, accumulating knowledge on risk factors of ACL injury is believed to be slowed. Many of these studies are too highly focused and do not – or are not formulated to – consider multiple risk factors.
Towards a better understanding of non-contact ACL injury determinants
Non-contact ACL injury biomechanics research requires the use of many modalities, data from disparate sources, and many specialized software tools [89]. A possible and coupled study approach should leverage on the advantages of existing non-contact ACL injury study approaches. The central aim of this potential study approach should be to provide an enabling tool to better capture the many variables, constraints, unknowns, uncertainty, and variability entailed in the complex problem of determining how and why the ACL gets injuried. This approach should be able to simultaneously capture the interaction of multiple forces, risk factors, and other parameters that may contribute to ACL injury in a seamless automated manner. This approach also should aim to provide information that can connect the cause and effect relationships between identified risk factors and injuries.
It is believed that despite its challenges, through tradeoff, the computational modeling approach of the human lower extremity combined with rigid body modeling when validated with experiments, as well as, existing qualitative study approaches, may be a more suitable methodology to determine risk factors for ACL injury. Such an approach allows for countless number and types of tests to be performed due to its virtual nature. Such an approach will allow for virtual experimentation which has significant implication for cost reduction through reduced equipment needs, number of subjects required for testing, and also time for testing. Nonetheless, a standalone detailed validated 3D musculoskeletal FE model of the lower extremity may not be able to identify risk factors for non-contact ACL injury. Due to immense variability in hard and soft tissue geometry, variability in hard and soft material properties, and variability in experimental data; a technique to handle such variability is needed. In addition, the numerous risk factors which are simultaneously at play during an ACL injury event, the numerous constraints, the high dimensionality, great interdependencies, and temporal dependence, as well as, the unknowns about kinematics and kinetics at time of injury, demands the utilization of an optimization routine.
Classical optimization techniques such as the Newton Raphson search method cannot be applied to the problem of how and why non-contact ACL injuries occur because of the absence of a polynomial type function, and more importantly it cannot handle many design parameters in a large domain. As an alternative, the authors believe a stochastic technique may be employed. As mentioned earlier, ACL injury is multifaceted with numerous parameters and constraints simultaneously at play to cause injury. Many present research methods limited since they are unable to simultaneously capture many objective functions, decision variables, and constraints in problem definition. There are few studies, to our best knowledge, where a tool or methodology is employed that lends itself to simultaneously determining effects of multiple parameters on ACL injury. The application of heuristic techniques to ACL injury biomechanics is relatively new and scare in ACL injury research, but provides an avenue to tackle problems involving many parameters, many constraints, and multiple objective functions. Mathematical programming and the Monte Carlo method [66, 90, 91] appears to be the dominant optimization approaches. Mathematical programming is used primarily to minimize the kinematics differences between subjects’ movement and simulated model movement. Monte Carlo method is used primarily in applications to evaluate the probability of random outcomes of human movement. Classical Monte Carlo methods are algorithms that randomly generate and retain the best solutions before going to the next search iteration. Although both mathematical programming and Monte Carlo methods have demonstrated their usefulness and effectiveness as a research tool, there are much more advanced and robust stochastic techniques. Among these techniques, are heuristic or artificially intelligent (AI) methods such as taboo search, simulated annealing, genetic algorithms, and artificial neural networks. For small search spaces, classical exhausted methods usually suffice; however, for larger search spaces, e.g. ACL injury mechanics studies, special AI techniques must be employed.
However, to gain a comprehensive view of factors contributing to ACL injury one needs to simultaneously address the multiple risk factors and be able to know when all extreme condition happen. The ability to fuse FE modeling with an AI technique that allows for automatic or semi-automatic data exchange may be necessary. A schematic outline of this approach is shown in Figure 2. To the authors’ best knowledge, there are no attempts to investigate risk factors for non-contact ACL injury via this approach. The authors believe that exploiting the strengths of various existing ACL injury study approaches in a combinatory manner may overshadow, yield improved information, and even negate the disadvantages encountered when each approach is employed on its own.
Figure 2- Possible approach to better understand risk factors to ACL
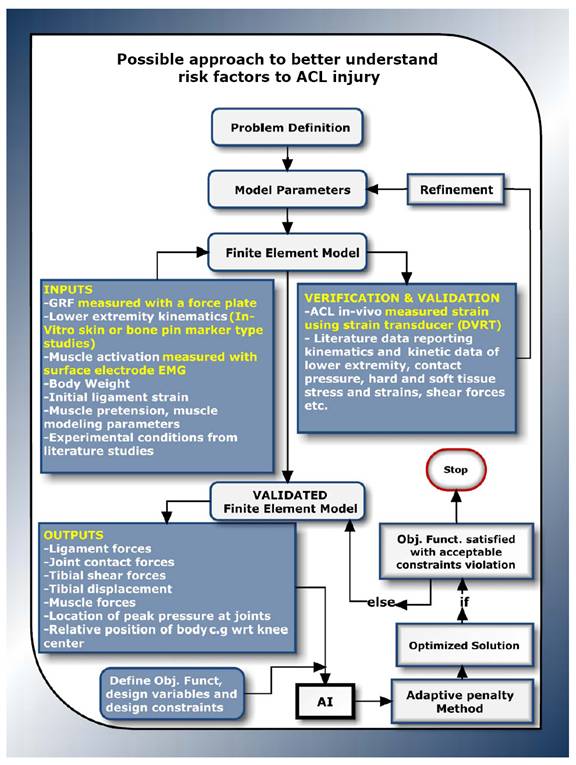
Summary and Conclusions
The general inherent and overarching challenges to better understand risk factors to non-contact ACL injury were presented. The precise combinations of risk factors that endanger the ACL are not completely understood. Studies to date only provide estimates, rather than precise measurements of knee kinematics at the time of injury. The mechanisms of ACL injury is very complex and may involve the concurrent interaction of multiple risk factors. The effect of such interaction on ACL injury is unknown. Moreover, determining the risk factors of ACL injury is difficult given many risk factors act at the same time to cause injury. Consequently, it is presently not known which prevention program component and risk factor is the key element in preventing ACL injuries, or how they work. No study to date has provided conclusive evidence that any of the implicated risk factors of non-contact ACL injuries progress into actual causes of injuries. The risk factors ordered in terms of risk level (e.g. high, medium, low) to cause ACL injury are also unknown.
This review is geared towards highlighting the present challenges to obtaining a better understanding of the risk factors of non-contact ACL injury. The ultimate goal of understanding ACL injury causation is to improve prevention and training strategies, as well as, improve clinical diagnosis and treatment of different injuries and disorders of diarthordial joints. Based on this review, it is clear that in spite of significant amount of research related to ACL injury, there are still many open questions about injury causation and conflicting views as to which factor most contributes to risk of non-contact ACL injury. There is a great need to develop new research methods capable of addressing the myriad of factors, high dimensionality, and great interdependencies involved in non-contact ACL injury.
Competing interest
The authors declare that they have no competing interests.
Authors’ contribution
NA was responsible for conception, writing, revising, and final approval of the manuscript, while GR was responsible for reviewing, drafting, and final approval of the manuscript.
Reference :
1. Barry PB, Dean GS, John AF, Jr., William EG, Jr.: Mechanisms of anterior cruciate ligament injury. Orthopedics 2000, 23:573-578.
2. Olsen OE, Myklebust G, Engebretsen L, Bahr R: Injury Mechanisms for Anterior Cruciate Ligament Injuries in Team Handball: A Systematic Video Analysis. In American Journal of Sports Medicine, vol. 32. pp. 1002: AOSSM; 2004:1002.
3. Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, Garrick JG, Hewett TE, Huston L, Ireland ML, et al: Noncontact Anterior Cruciate Ligament Injuries: Risk Factors and Prevention Strategies. In Journal of the American Academy of Orthopaedic Surgeons, vol. 8. pp. 141-150; 2000:141-150.
4. Yu BPD, Kirkendall DTPD, Garrett WEJMDPD: Anterior Cruciate Ligament Injuries in Female Athletes: Anatomy, Physiology, and Motor Control. Sports Medicine & Arthroscopy Review The Female Athlete 2002, 10:58-68.
5. Gottlob CAMD, Baker CLJMD, Pellissier JMP, Colvin LP: Cost Effectiveness of Anterior Cruciate Ligament Reconstruction in Young Adults. SO - Clinical Orthopaedics & Related Research October 1999;367:272-282 1999.
6. Huston LJ, Greenfield M, Wojtys EM: Anterior Cruciate Ligament Injuries in the Female Athlete: Potential Risk Factors. In Clinical Orthopaedics and Related Research, vol. 372. pp. 50; 2000:50.
7. Daniel DM, Fristschy, D.: Anterior cruciate ligament injuries Orthopaedic Sports Medicine: Principles and Practice 1994.
8. Whiting WC, Zernicke RF: Biomechanics of musculoskeletal injury. Human Kinetics Champaign, IL; 1998.
9. McLean SG, Walker K, Ford KR, Myer GD, Hewett TE, van den Bogert AJ: Evaluation of a two dimensional analysis method as a screening and evaluation tool for anterior cruciate ligament injury. In British Journal of Sports Medicine, vol. 39. pp. 355; 2005:355.
10. Meeuwisse WH: Assessing Causation in Sport Injury: A Multifactorial Model. In Clinical Journal of Sport Medicine, vol. 4. pp. 166-170; 1994:166-170.
11. Fleming BC, Renstrom PA, Beynnon BD, Engstrom B, Peura GD, Badger GJ, Johnson RJ: The effect of weightbearing and external loading on anterior cruciate ligament strain. Journal of Biomechanics 2001, 34:163-170.
12. Pflum MA, Shelburne KB, Torry MR, Decker MJ, Pandy MG: Model Prediction of Anterior Cruciate Ligament Force during Drop-Landings. Medicine & Science in Sports & Exercise 2004, 36:1949-1958.
13. Fleming BC, Beynnon BD, Churchill DL, Webster JD, Renstrom PA: The effect of weightbearing and bracing in the anterior cruciate ligament deficient knee Transaction orthopaedic Research Society 1999, 28.
14. Ziegler J, Pandy MG: A computational model for determining muscle–ligament interactions at the knee during movement. In Computational Medicine. pp. 532–568; 1995:532–568.
15. Lephart SM, Abt JP, Ferris CM: Neuromuscular contributions to anterior cruciate ligament injuries in females. In Current Opinion in Rheumatology, vol. 14. pp. 168-173; 2002:168-173.
16. Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, DeMaio M, Dick RW, Engebretsen L, Garrett WE, Jr., Hannafin JA, et al: Understanding and Preventing Noncontact Anterior Cruciate Ligament Injuries: A Review of the Hunt Valley II Meeting, January 2005. In American Journal of Sports Medicine, vol. 34. pp. 1512-1532; 2006:1512-1532.
17. Beynnon BD, Fleming BC: Anterior cruciate ligament strain in-vivo: A review of previous work. Journal of Biomechanics 1998, 31:519-525.
18. McConkey JP: Anterior cruciate ligament rupture in skiing: A new mechanism of injury. In The American Journal of Sports Medicine, vol. 14. pp. 160-164; 1986:160-164.
19. Markolf KL, O'Neill G, Jackson SR, McAllister DR: Effects of Applied Quadriceps and Hamstrings Muscle Loads on Forces in the Anterior and Posterior Cruciate Ligaments. In American Journal of Sports Medicine, vol. 32. pp. 1144-1149; 2004:1144-1149.
20. Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SLY: The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. Journal of Biomechanics 1999, 32:395-400.
21. Beynnon BD, Fleming BC, Johnson RJ, Nichols CE, Renstrom PA, Pope MH: Anterior Cruciate Ligament Strain Behavior During Rehabilitation Exercises In Vivo. The American Journal of Sports Medicine 1995, 23:24-34.
22. Aune AK, Cawley PW, Ekeland A: Quadriceps Muscle Contraction Protects the Anterior Cruciate Ligament During Anterior Tibial Translation. In The American Journal of Sports Medicine, vol. 25. pp. 187-190; 1997:187-190.
23. DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W: Aggressive Quadriceps Loading Can Induce Noncontact Anterior Cruciate Ligament Injury. In The American Journal of Sports Medicine, vol. 32. pp. 477-483; 2004:477-483.
24. Isaac DL, Beard DJ, Price AJ, Rees J, Murray DW, Dodd CAF: In-vivo sagittal plane knee kinematics: ACL intact, deficient and reconstructed knees. The Knee 2005, 12:25-31.
25. Solomonow M, Baratta R, D'Ambrosia R: The role of the hamstrings in the rehabilitation of the anterior cruciate ligament-deficient knee in athletes. In Sports Med, vol. 7. pp. 42-48; 1989:42-48.
26. Shelburne KB, Pandy MG: Determinants of cruciate-ligament loading during rehabilitation exercise. Clinical Biomechanics 1998, 13:403-413.
27. Simonsen EB, Magnusson SP, Bencke J, Naesborg H, Havkrog M, Ebstrup JF, Sorensen H: Can the hamstring muscles protect the anterior cruciate ligament during a side-cutting maneuver? In Scandinavian Journal of Medicine & Science in Sports, vol. 10. pp. 78-84: Blackwell Synergy; 2000:78-84.
28. Fleming BC, Renstrom PA, Ohlen G, Johnson RJ, Peura GD, Beynnon BD, Badger GJ: The gastrocnemius muscle is an antagonist of the anterior cruciate ligament. Journal of Orthopaedic Research 2001, 19:1178-1184.
29. Durselen L, Claes L, Kiefer H: The Influence of Muscle Forces and External Loads on Cruciate Ligament Strain. In The American Journal of Sports Medicine, vol. 23. pp. 129-136; 1995:129-136.
30. Shelburne KB, Pandy MG: A musculoskeletal model of the knee for evaluating ligament forces during isometric contractions. Journal of Biomechanics 1997, 30:163-176.
31. Olsen OE, Myklebust G, Engebretsen L, Holme I, Bahr R: Relationship between floor type and risk of ACL injury in team handball. In Scandinavian Journal of Medicine & Science in Sports, vol. 13. pp. 299-304; 2003:299-304.
32. Lambson RB, Barnhill BS, Higgins RW: Football Cleat Design and Its Effect on Anterior Cruciate Ligament Injuries: A Three-Year Prospective Study. In The American Journal of Sports Medicine, vol. 24. pp. 155-159; 1996:155-159.
33. Chaudhari AM, Andriacchi TP: The mechanical consequences of dynamic frontal plane limb alignment for non-contact ACL injury. Journal of Biomechanics 2006, 39:330-338.
34. Smith J, Szczerba JE, Arnold BL, Perrin DH, Martin DE: Role of Hyperpronation as a Possible Risk Factor for Anterior Cruciate Ligament Injuries. In Journal of Athletic Training, vol. 32. pp. 25: National Athletic Trainers Association; 1997:25.
35. Anderson FC, Pandy MG: Individual muscle contributions to support in normal walking. Gait & Posture 2003, 17:159-169.
36. Ireland ML: The female ACL: why is it more prone to injury? In Orthopedic Clinics of North America, vol. 33. pp. 637-651: Elsevier; 2002:637-651.
37. Hashemi J, Chandrashekar N, Jang T, Karpat F, Oseto M, Ekwaro-Osire S: An Alternative Mechanism of Non-contact Anterior Cruciate Ligament Injury During Jump-landing: In-vitro Simulation. In Experimental Mechanics, vol. 47. pp. 347-354: Springer; 2007:347-354.
38. Yu B, Lin C-F, Garrett WE: Lower extremity biomechanics during the landing of a stop-jump task. Clinical Biomechanics 2006, 21:297-305.
39. McLean SG, Huang X, van den Bogert AJ: Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: Implications for ACL injury. In Clinical Biomechanics, vol. 20. pp. 863-870: Elsevier; 2005:863-870.
40. Hewett TE, Myer GD, Ford KR: Anterior Cruciate Ligament Injuries in Female Athletes: Part 1, Mechanisms and Risk Factors. In American Journal of Sports Medicine, vol. 34. pp. 299: AOSSM; 2006:299.
41. Meakin JR, Shrive NG, Frank CB, Hart DA: Finite element analysis of the meniscus: the influence of geometry and material properties on its behaviour. In Knee, vol. 10. pp. 33-41; 2003:33-41.
42. Zeiss J, Paley K, Murray K, Saddemi SR: Comparison of Bone Contusion Seen by MRI in Partial and Complete Tears of the Anterior Cruciate Ligament. In Journal of Computer Assisted Tomography, vol. 19. pp. 773; 1995:773.
43. Li G, Rudy TW, Allen C, Sakane M, Woo SL, hyphen, Y: Effect of combined axial compressive and anterior tibial loads on in situ forces in the anterior cruciate ligament: A porcine study. In Journal of Orthopaedic Research, vol. 16 pp. 122 - 127: Wiley Subscription Services, Inc., A Wiley Company; 1998:122 - 127.
44. Liu W, Maitland ME: Influence of Anthropometric and Mechanical Variations on Functional Instability in the ACL-Deficient Knee. In Annals of Biomedical Engineering, vol. 31. pp. 1153-1161: Springer; 2003:1153-1161.
45. Renstrom P, Arms SW, Stanwyck TS, Johnson RJ, Pope MH: Strain within the anterior cruciate ligament during hamstring and quadriceps activity. In The American Journal of Sports Medicine, vol. 14. pp. 83-87; 1986:83-87.
46. Krosshaug T, Andersen TE, Olsen OEO, Myklebust G, Bahr R: Research approaches to describe the mechanisms of injuries in sport: limitations and possibilities. In British Journal of Sports Medicine, vol. 39. pp. 330-339; 2005:330-339.
47. Berns GS, Hull ML, Patterson HA: Strain in the anteromedial bundle of the anterior cruciate ligament under combination loading. In Journal of Orthopaedic Research, vol. 10. pp. 167-176; 1992:167-176.
48. Woo SLY, Abramowitch SD, Kilger R, Liang R: Biomechanics of knee ligaments: injury, healing, and repair. Journal of Biomechanics 2006, 39:1-20.
49. Cerulli G, Benoit DL, Lamontagne M, Caraffa A, Liti A: In vivo anterior cruciate ligament strain behaviour during a rapid deceleration movement: case report. Knee Surgery, Sports Traumatology, Arthroscopy 2003, 11:307-311.
50. Ahmed AM, Burke DL, Duncan NA, Chan KH: Ligament tension pattern in the flexed knee in combined passive anterior translation and axial rotation. In Journal of Orthopaedic Research, vol. 8. pp. 854 - 867: Wiley Subscription Services, Inc., A Wiley Company; 1992:854 - 867.
51. An KN, Berflund, L, Cooney, W P, Chao E Y S, Kovacevic N.: Direct in-vivo tendon force measurement system. Journal of Biomechanical Engineering 1990, 23:1269-1271.
52. Meijer RCMB, Huiskes R, Kauer JMG: A stereophotogrammetric method for measurements of ligament structure. Journal of Biomechanics 1989, 22:177-179.
53. Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA: The Relationship between Quadriceps Muscle Force, Knee Flexion, and Anterior Cruciate Ligament Strain in an In Vitro Simulated Jump Landing. American Journal of Sports Medicine 2006, 34:269-274.
54. Mommersteeg TJA, Huiskes R, Blankevoort L, Kooloos JGM, Kauer JMG: An inverse dynamics modeling approach to determine the restraining function of human knee ligament bundles. In Journal of Biomechanics, vol. 30. pp. 139-146: Elsevier; 1997:139-146.
55. Haimes JL, Wroble RR, Grood ES, Noyes FR: Role of the Medial Structures in the intact and Anterior Cruciate Ligament-Deficient Knee: Limits of Motion in the Human Knee. In The American Journal of Sports Medicine,, vol. 22. pp. 402: AOSSM; 1994:402.
56. Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GAM, Slauterbeck JL: Combined knee loading states that generate high anterior cruciate ligament forces. In Journal of Orthopaedic Research. pp. 930 - 935: Wiley Subscription Services, Inc., A Wiley Company; 1995:930 - 935.
57. Benoit DL, Ramsey DK, Lamontagne M, Xu L, Wretenberg P, Renstrom P: Effect of skin movement artifact on knee kinematics during gait and cutting motions measured in vivo. In Gait Posture, vol. 24. pp. 152-164: Elsevier; 2006:152-164.
58. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W: Abnormal Rotational Knee Motion During Running After Anterior Cruciate Ligament Reconstruction. In American Journal of Sports Medicine, vol. 32. pp. 975: AOSSM; 2004:975.
59. Crowninshield R, Pope MH, Johnson RJ: An analytical model of the knee. In Journal of Biomechanics, vol. 9. pp. 397-405; 1976:397-405.
60. Hefzy MS, Cooke TDV: Review of Knee Models: 1996 Update. In Appl Mech Rev, vol. 49. pp. 187–193; 1996:187–193.
61. Andriacchi TP, Mikosz RP, Hampton SJ, Galante JO: Model studies of the stiffness characteristics of the human knee joint. In Journal of biomechanics, vol. 16. pp. 23-29; 1983:23-29.
62. Limbert G, Taylor M, Middleton J: Three-dimensional finite element modelling of the human ACL: simulation of passive knee flexion with a stressed and stress-free ACL. Journal of Biomechanics 2004, 37:1723-1731.
63. Song Y, Debski RE, Musahl V, Thomas M, Woo SLY: A three-dimensional finite element model of the human anterior cruciate ligament: a computational analysis with experimental validation. In Journal of Biomechanics, vol. 37. pp. 383-390: Elsevier; 2004:383-390.
64. Gabriel M, Wong E, Woo S, Yagi M, Debski R: Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. In Journal of Orthopaedic Research, vol. 22. pp. 85-89; 2004:85-89.
65. Fregly BJ, Bei Y, Sylvester ME: Experimental evaluation of an elastic foundation model to predict contact pressures in knee replacements. In Journal of Biomechanics, vol. 36. pp. 1659-1668: Elsevier; 2003:1659-1668.
66. McLean SG, Su A, van den Bogert AJ: Development and Validation of a 3-D Model to Predict Knee Joint Loading During Dynamic Movement. Journal of Biomechanical Engineering 2004, 125:864-874.
67. Suggs J, Wang C, Li G: The effect of graft stiffness on knee joint biomechanics after ACL reconstruction––a 3D computational simulation. In Clinical Biomechanics, vol. 18. pp. 35-43: Elsevier; 2003:35-43.
68. Pandy MG, Sasaki K, Kim S: A Three-Dimensional Musculoskeletal Model of the Human Knee Joint. Part 1: Theoretical Construction. In Computer Methods in Biomechanics and Biomedical Engineering, vol. 1. pp. 87-108: Taylor & Francis; 1997:87-108.
69. Anderson FC, Pandy MG: Static and dynamic optimization solutions for gait are practically equivalent. In Journal of Biomechanics, vol. 34. pp. 153-161: Elsevier; 2001:153-161.
70. Viceconti M, Olsen S, Nolte LP, Burton K: Extracting clinically relevant data from finite element simulations. In Clinical Biomechanics, vol. 20. pp. 451-454: Elsevier; 2005:451-454.
71. Payne CB, Bird AR: Teaching clinical biomechanics in the context of uncertainty. In Journal of the American Podiatric Medical Association, vol. 89. pp. 525-530; 1999:525-530.
72. Peña E, Calvo B, Martínez MA, Palanca D, Doblaré M: Finite element analysis of the effect of meniscal tears and meniscectomies on human knee biomechanics. In Clinical Biomechanics,, vol. 20. pp. 498-507: Elsevier; 2005:498-507.
73. Au AG, Raso VJ, Liggins AB, Otto DD, Amirfazli A: A three-dimensional finite element stress analysis for tunnel placement and buttons in anterior cruciate ligament reconstructions. Journal of Biomechanics 2005, 38:827-832.
74. Moglo KE, Shirazi-Adl A: Cruciate coupling and screw-home mechanism in passive knee joint during extension–flexion. In Journal of Biomechanics, vol. 38. pp. 1075-1083: Elsevier; 2005:1075-1083.
75. Ramaniraka NA, Terrier A, Theumann N, Siegrist O: Effects of the posterior cruciate ligament reconstruction on the biomechanics of the knee joint: a finite element analysis. In Clinical Biomechanics, vol. 20. pp. 434-442: Elsevier; 2005:434-442.
76. Peña E, Calvo B, Martínez MA, Palanca D, Doblaré M: Computational Modelling of Diarthrodial Joints. Physiological, Pathological and Pos-Surgery Simulations. Arch Comput Methods Eng 2007, 14:47-91.
77. Ramaniraka NA, Saunier P, Siegrist O, Pioletti DP: Biomechanical evaluation of intra-articular and extra-articular procedures in anterior cruciate ligament reconstruction: A finite element analysis. In Clinical Biomechanics, vol. 22. pp. 336-343: Elsevier; 2007:336-343.
78. Sawatari T, Tsumura H, Iesaka K, Furushiro Y, Torisu T: Three-dimensional finite element analysis of unicompartmental knee arthroplasty––the influence of tibial component inclination. In J Orthop Res, vol. 23. pp. 549-554; 2005:549-554.
79. Elias JJ, Cosgarea AJ: Computational Modeling: An Alternative Approach for Investigating Patellofemoral Mechanics. vol. 15. pp. 89-94: Sports Medicine and Arthroscopy Review RAVEN PRESS, LTD.; 2007:89-94.
80. Beillas P, Papaioannou G, Tashman S, Yang KH: A new method to investigate in vivo knee behavior using a finite element model of the lower limb. Journal of Biomechanics 2004, 37:1019-1030.
81. Zhiheng J, Zhijiang D, Monan W: A Novel Finite Element Method based Biomechanical Model for HIT-Robot Assisted Orthopedic Surgery System. In Engineering in Medicine and Biology Society, 2006 EMBS '06 28th Annual International Conference of the IEEE. 2006: 1735-1738.
82. Murphy DF, Connolly DAJ, Beynnon BD: Risk factors for lower extremity injury: a review of the literature. In British Journal of Sports Medicine, vol. 37. pp. 13; 2003:13.
83. Horton MG, Hall TL: Quadriceps femoris muscle angle: normal values and relationships with gender and selected skeletal measures. In Phys Ther vol. 69. pp. 897-901; 1989:897-901.
84. Tillman MD, Smith KR, Bauer JA, Cauraugh JH, Falsetti AB, Pattishall JL: Differences in three intercondylar notch geometry indices between males and females: a cadaver study. In Knee, vol. 9. pp. 41-46; 2002:41-46.
85. Wojtys EM, Huston LJ, Lindenfeld TN, Hewett TE, Greenfield M: Association Between the Menstrual Cycle and Anterior Cruciate Ligament Injuries in Female Athletes. In The American Journal of Sports Medicine, vol. 26. pp. 614: AOSSM; 1998:614.
86. NIH: The National Library of Medicine's Visible Human Project. http://wwwnlmnihgov/research/visible/visible_humanhtml 2000.
87. Jan SVS: The VAKHUM project: virtual animation of the kinematics of the human. In Theoretical Issues in Ergonomics Science, vol. 6. pp. 277-279: Taylor & Francis; 2005:277-279.
88. Viceconti M, Ansaloni M, Baleani M, Toni A: The muscle standardized femur: a step forward in the replication of numerical studies in biomechanics. In Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine, vol. 217. pp. 105-110: Prof Eng Publishing; 2003:105-110.
89. Viceconti M, Taddei F, Montanari L, Testi D, Leardini A, Clapworthy G, Van Sint Jan S: Multimod Data Manager: A tool for data fusion. vol. 87. pp. 148-159: Elsevier; 2007:148-159.
90. Blankevoort L, Huiskes R: Validation of a three-dimensional model of the knee. Journal of Biomechanics 1996, 29:955-961.
91. Scott GM, Xuemei H, Anne S, Antonie JvdB: Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clinical biomechanics (Bristol, Avon) 2004, 19:828-838
|




