|
Abstract:
Purpose: To further understand the viability of the non-vascularized
bone graft and the origin of the bone-forming cells, we have
developed a novel mouse femur model that permits direct-viewing
analysis of bone graft healing.
Materials and Methods: Critical size defects were
prepared in one side of femur of 15 C57BL/6 mice and repaired by
non-vascularized femur with intact periosteum and parts of
muscles from isogenous GFP transgenic C57BL/6 mice. Sacrifice
was performed 3 days, 1, 2, 3, and 4 weeks after surgery for
histological and fluorescence microscopy analysis.
Results: Healing process of the transplants is similar to
that of bone fracture. The osteoblasts and newly formed
trabeculae which were beneath the grafted periosteum showed
green fluorescent protein (GFP) expression, which was strongly
expressed in the transplanted muscles.
Conclusion: These data demonstrate that part of non-vascularized
grafted bone can keep viability and has the potential of
osteogenesis, indicating an important role for the grafted
periosteum and bone in new bone formation
J.Orthopaedics 2008;5(3)e15
Keywords:
GFP transgenic mice; isograft; non-vascularized bone graft.
Introduction:
Non-vascularized
bone graft, as an effective method for the repair of bone
defects, is widely used in the world. Conventional view of
incorporation of non-vascularized bone graft to the recipient is
called creeping substitution.1
The grafts will finally be totally replaced by the host and all
bone-forming cells originate from the recipients.2
However, some researchers hold a contrary opinion. Ray
3used a
radioactive tracer as a bone label to perform bone
transplantation between highly inbred mice. And Arora
4 identified sex chromatin
following sex-mismatched bone transplantation between highly
inbred rabbits. They found that not all the grafted bone
suffered from creeping substitution, but a few parts of them
could keep its viability. Kakinoki
5 transferred non-vascularized tibia grafts from
male to female rats, then extracted genomic DNA and made Sry-specific
PCR detection. Their results demonstrated that a small number of
grafted bone survived and proliferated over time. However, these
methods have low sensitivity, much complication; they have
provided limited understanding of transplanted cells.
Green
fluorescent protein (GFP) is a 27-KD protein, originated in
jellyfish. It provides a technically assessment of transgene
activity and can be viewed as a real-time image in living
tissues. Recently Jiang
6
reported that GFP activity can be preserved in paraffin
sections for standard fluorescence microscopy. Thus, itís
hypothesized that GFP transgenic mouse as transplantation donors
would be an easier way to determine the origin of newly forming
cells and comprehend clearly the behavior of grafted cells
during the repair process of bone defects.
In the
present study, we transplanted non-vascularized femur grafts
from
GFP transgenic mice to its isogenous mice. Fluorescence
microscopic and histological assay were performed at 3 day, 1,
2, 3 and 4 weeks after surgery, to identify the viability of the
grafted bone and the origin of the bone-forming cells. This
study may be of importance in the future development of clinical
methods to reconstruct large mandible defects using non-vascularized
bone grafts with dental implants at the same time.
Material and Methods :
The study
procedure was approved by the animal center of Shaníxi province.
All animal experiments were performed according to the
guidelines of the animal committee of the Fourth Military
Medical University.
Surgery
Procedure
Fifteen
young adult male C57BL/6 mice (weighing 30-50g) and fifteen
12-week male GFP transgenic C57BL/6 mice (weighing 25-30g) were
used in this study. All surgeries were performed under general
anesthesia with 1%
sodium pentobarbital by intraperitoneal injection. A
standardized metaphyseal bone defect of the femur (5mm long,
full thickness) was created in C57BL/6 mouse. Then a
5mm-long-femur with intact periosteum and parts of muscles was
harvested from GFP transgenic C57BL/6 mouse. The graft was
carefully placed into the defect of the C57BL/6 mouse and fixed
with sutures. Wound was closed in layers and fixed with bandage
(Ex vivo time of the graft was less than 15 minutes). Animals
were sacrificed at 3 days, 1, 2, 3 and 4weeks after surgery
respectively and the grafts were carefully harvested and fixed
immediately into 10% phosphate-buffered neutral formaldehyde for
24 hours.
Histology
For
histology, the specimens were demineralized in 0.1M EDTA for 2
weeks, dehydrated in graded ethanol solutions, embedded in
paraffin. Serial sections were cut in 4-μm-thick at different
levels sufficiently far apart (about 200μm) to avoid replicate
sampling of a single surface event. Sections were then mounted
on glass slides and stored at -20℃ away from light. Finally,
some sections were stained with Hematoxylin & Eosin and Masson
trichrome, whereas others were left unstained for evaluation
under fluorescence microscopy.
Fluorescence
microscopy
All
sections were observed under fluorescence microscopy. The
average exposure time was 1-1.5 sec and the excitation light is
488nm~530nm blue light.
Results :
All animals
remained healthy during the healing period and healed
uneventfully. Infection and immunological rejection were not
observed.
Histology
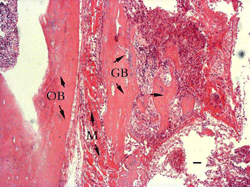
3 days:
There were
lots of blood clots and inflammatory cells in the grafted areas.
Transplanted bone was partly absorbed and a thin layer of newly
formed immature trabecular was on the surface of them (Figure
1).
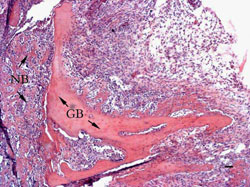
1 week:
Blood clots had been replaced by vascularized granulation
tissues which extended all the way to the grafted bed.
The
union between the grafted bone and recipient bone was full of
mesenchymal cells undergoing chondrocytic differentiation.
Extensive bone absorption was observed at the periphery of the
bone graft indicated by the numbers of osteoclasts (Figure 2).
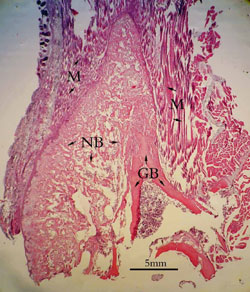
2 weeks:
The
grafted areas could be divided into five layers according to
different tissues: grafted muscles, grafted periosteum, newly
formed cartilage, newly formed trabeculae and grafted bone.
Muscle fibers interlacing with each other were in the outside of
the grafted areas. Their cellular nuclei were on the margins of
the fibers. Connecting with muscle fibers, periosteum was intact
and numbers of mesenchymal cells aggregated in its germinal
layer. Beneath the periosteum
there were lots of chondrocytes which seemed big and more
karyokinesis. Newly formed woven trabeculae occupied nearly two
thirds of grafted areas and numerous osteoblasts lined around
them. One third of the cortex of grafted bone had been absorbed
and new bone formation occurred on the superficial of the
remains. Grafted bone marrow was almost intact and within it
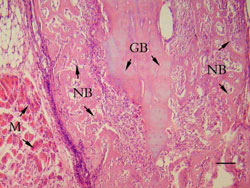
lots of lymphocyte and leukocytes got together (Figure 3).
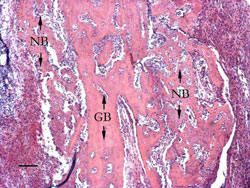
3 weeks:
The remained grafted bone began to integrate with newly formed
mineralized trabecular. New medullary cavity occurred in the
area initially occupied by the bone grafts (Figure 4).
4 weeks:
Newly formed bone incorporated to the grafted bone and itís
difficult to distinguish them (Figure 5).
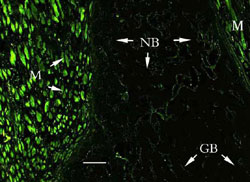
Fluorescence
microscopy
Bright
green light was found in transplanted muscles all the time under
fluorescent microscopy. The osteoblasts and newly formed
trabeculae, which were beneath the grafted periosteum, also
showed GFP expression (Figure 6).
Discussion :
Although
the theory of
creeping substitution has been introduced for more than one hundred
years, there are still two questions not very clear: Does all
the grafted bone be replaced by the newly formed bone? And where
are the osteoblasts or osteocytes in the new forming bone from?
7, 8
By now, large number of researches support that parts of
the grafted bone will survive and have the ability of
osteogenesis.9, 10, 11, 12
In this study, we investigated the viability of the non-vascularized
grafted bone and its ability of new bone formation using
isografting from fluorescence specific GFP transgenic mice to
its isogenous normal mice. Our results suggest that the
transplants can keep their viability and have the ability of
osteogenesis. Although large part of the grafted bone was
absorbed, the remains still kept its structure. Transplanted
muscles and periosteum showed their viability by positive
expression of green lights. Green lights in the newly formed
trabeculae and osteoblasts revealed that bone forming cells were
from the transplants. Thus our results indicate that the grafted
bone and periosteum play an important role in the process of new
bone formation, and that the transplant can keep its viability
during the reparative process.
Owing to the
early revascularization, Transplanted bone, periosteum and
muscles could keep their vitality and medullary cavity of
grafted bone could keep its structure, as the femur cortex of
mouse is thin, which facilitates blood vessels going through.
Early revascularization was established by the blood-abundant
recipient bed and the transplanted soft tissues which can get
blood easier than bone. Femur cortex of mouse is about
1mm thick,
through which blood vessels go easily. No fixation in
medullary cavity could kept its structure and conduce to
revascularization. Healing process of transplants is similar to
the healing process of bone fracture.
In this
study, we used GFP transgenic C57BL/6 mice as donor animals.
These animals are very special with green muscles and bones in
the sunlight and green eyes and ears under violet light.
Histologically, muscles show bright green light under
fluorescence microscopy. To bone, some researchers once worried
that the demineralization might destroy the expression of GFP.
Recently,
Jiang
6 has detected positive
green expression in demineralized bone sections. Itís very
interesting that positive expression only confine in osteoblasts
and at the margin of the bone instead of the whole bone. Our
study used 0.1M EDTA for 2 weeksí demineralization, and the
results indicated that GFP can be preserved in paraffin sections
for standard fluorescence microscopy. Itís very helpful to use
GFP transgenic mouse to be an animal model to study non-vascularized
bone graft.
Conclusion:
In summary,
the present study demonstrated that parts of non-vascularized
grafted bone can keep viability and has the potential of
osteogenesis. The grafted periosteum and bone play an important
role in new bone formation. GFP transgenic mouse may be a
helpful animal to identify the cellular origin or cellular
turnover in bone transplantation.
Acknowledegments
The authors
would like to thank Mr. Ya-gang Wei for his valuable assistance
in preparing the histological sections and works in relation to
the histomorphometry, Prof. Lian-jia Yang and Prof. Shao-zhong
Dong for their professional help in histological observations
and Doc. Li-An Wu and Prof. Qin Ma for their kind help in
English writing.
Reference :
|








