|
Abstract:
Study design: A retrospective review of image data was
performed in 123 patients with congenital scolisis.
Objective: To assess the vertebral deformity and intraspinal
anomaly and try to establish a classification for genetic study
of congenital scoliosis.
Summary of Backgroud Data: Three-dimensional reconstructions
facilitate visualization and evaluation of vertebral anomaly in
congenital scoliosis. Up to date, an appropriate classification
of congenital scoliosis is still unavailable for genetic study.
Method: We examined all the components of vertebrae to find the
entire anomalies by three-dimensional CT. We classified these
anomalies according to formation failure, segmentation failure
or mixed type, solitary malformation or multiple malformation,
and anterior malformation, posterior malformation or
anteroposterior malformation. The intraspinal anomaly was
detected by myelography and CT myelography.
Results: 123 patients consisted of 28 patients with formation
failure, 43 patients with segmentation failure, and 52 patients
with combination of formation failure and segmentation failure.
The incidence of intraspinal anomaly was 31.7%.
Conclusion: The combination of three-dimension reconstructions
with CT myelography can provide a complete understanding of both
vertebral deformity and intraspinal anomaly before surgery. The
classification, which based on three-dimensional
reconstructions, is all-round and reliable.
J.Orthopaedics 2007;4(4)e2
index.htm
Introduction:
Congenital scoliosis is due to the presence of vertebral anomalies
that cause an imbalance in the longitudinal growth of the spine.
The classification of congenital scoliosis proposed by Moe and
Winter et al [1,2]classified the vertebral anomalies according
to morphologic characteristics on AP and lateral plain images
into formation failure, segmentation failure, and a mixed type,
and this classification has been widely accepted. However, this
classification can be evaluated only by the vertebral body and
the pedicle of the vertebral arch in the malformed vertebrae,
and it is impossible to perform an all-round evaluation of the
morphology of the malformed vertebrae including the posterior
components only based on the plain images. Furthermore, the
vertebral rotation associated with scoliosis and the vertebral
overlapping of severe kyphoscoliosis on plain images make it
difficult to identify the vertebral pedicles, which is important
to discriminate between hemivertebrae and wedge vertebrae, and
evaluate the morphology of vertebral bodies . So the
classification system needs to be revised to provide more
detailed information of the anomalies.
Three-dimension CT scans are playing a more and more important role in
scoliosis diagnosis and classification. The detailed observation
of the posterior and anterior components of malformed vertebrae,
which can not be obtained from plain images and only be viewed
during the operation before, has become possible with the aid of
three-dimension CT scans. Many studies have shown the advantages
of CT application in the diagnosis and treatment of congenital
scoliosis, but it is a pity that no new classification based on
the detailed CT data was put forth. Since CT myelography (CTM)
and three-dimension CT reconstructions have been routinely
applied in the preoperative evaluation of scoliosis in our
hospital, so we did some work to classify the congenital
scoliosis on the basis of combination of plain images and 3D CT
reconstructions
Material and Methods :
A retrospective review was performed of patients with congenital
scoliosis cared for at Peiking Union Medical College Hospital
from 2005 through 2007. Institutional Review Board approval was
obtained. 123 patients with congenital scoliosis were diagnosed
and treated in the spine center. The initial diagnosis was made
based on the vertebral anomalies on the plain images and was
further verified after the CTM and 3D reconstructions were
performed. The subjects that are diagnosed as the known
syndromes, such as hemifacial microsomia, Alagille, Jarcho-Levin,
Klippel-Feil, Goldenhar, Joubert, basal cell nevus, trisomy 18,
diabetic embryopathy, and VACTERL (vertebral, anal, cardiac,
tracheal, esophageal, renal, and limb) syndromes, were excluded
so that the data become relative simple to be analyzed. In this
group, the subjects consisted of 51 males and 72 females, and
their age at the time of admission to the hospital was between 2
and 25 years (mean, 13.5 years).
All the patients had standing anteroposterior and lateral views of the
spine from C1 to S1. The patients were subjected to myelography
followed by computerized axial tomography to detect the
associated intraspinal anomalies. The dye flowed up to the
occipitovertebral junction to rule out Arnold-Chiari
malformation, syringomyelia, etc.
Both the malformed vertebra (e) and its (their) adjacent vertebrae
were observed in detail by 3D CT. The morphology of each of
components, including vertebral body, vertebral pedicles, neural
arch, and the relationship between the anterior and posterior
components were evaluated.
The CT scanner is a helical scanner (Somatom Plus S or Somatom Plus 4;
Siemens; Erlangen, Germany). The slice thickness was 3 mm, and
3D images were displayed by volume rendering. Slices over the
entire area of the scoliosis, including the thorax cage, were
obtained. High quality three-dimensional CT images were then
generated by the CT technician for every 30° of rotation about
the longitudinal axis of the spine.
Although the classification of congenital scoliosis proposed by Moe
and Winter et al was simple and primitive, it was very helpful
to define the feature of the vertebral anomaly. Philip and
Robert et al had proposed some genes related to the vertebral
anomaly, which is the direction of our following research. So
here we made some modification of the classification for the
further genetic study. First, the patients were classified into
3 groups: formation failure group (FF), segmentation failure
group (SF) and mixed type group (MT). Anomaly of any part of the
vertebra was determined to be abnormal vertebra. The formation
failure group was further subdivided into the solitary
malformation group (SM), which exhibited only a single malformed
vertebra in the entire spine; and the multiple malformations
group (MM), which exhibited multiple malformed vertebrae. The
segmentation group was also subdivided into the solitary
malformation group (SM), in which only the consecutive two
vertebrae were involved; and the multiple malformations group
(MM), in which more than two vertebrae were involved. In MT
group, solitary malformation meant segmentation failure between
the malformed vertebra and one of the adjacent vertebrae, and
the rest were multiple malformations. Then each group was
subdivided into anterior malformation subgroup (AM), posterior
malformation subgroup (PM) and anteroposterior malformation
subgroup (APM).
Results :
Vertebral Deformity
Congenital scoliosis was more frequent in girls (male:female ratio
1:1.41). Associated kyphosis was seen in 61 (49.6%) patients.
The distribution of all the cases in three groups is showed in
Table 1. Of all the 123 patients, 29 cases were formation
failure,; 43 cases were segmentation failure, and 51 cases were
mixed type. The frequency of each involved segment was exhibited
in Fig. 1. In the FF group, most of the involved segments were
located between T8-L4 (76.4%), and there was no obvious peak. In
the SF group, 91.6% of the involved levels were located in the
thoracic segments. In the MT group, T7 was the most frequently
involved segment and the wave centered on T7 and decreased
caudally and rostrally. Intriguing, in our series C1-5 were free
of anomaly. The average lengths of involved vertebrae were 1.5,
5.0, and 5.3 in FF group, SF group and MT group respectively.
Table 1. The classification of 123 patients
|
|
FF |
|
SF |
|
MT |
|
|
SM |
MM |
Sum |
|
SM |
MM |
Sum |
|
SM |
MM |
Sum |
|
AM |
13 |
6 |
19 |
|
4 |
5 |
9 |
|
1 |
5 |
6 |
|
PM |
0 |
0 |
0 |
|
0 |
3 |
3 |
|
0 |
0 |
0 |
|
APM |
6 |
3 |
9 |
|
5 |
26 |
31 |
|
5 |
41 |
46 |
|
Sum |
19 |
9 |
|
|
9 |
34 |
|
|
6 |
46 |
|
|
Total |
28 |
|
43 |
|
52 |
Nineteen-nine patients (67.9%) were solitary malformation, and nine
patients (32.1%) were multiple malformations in the twenty-eight
patients of FF group. The multiple malformations were
consecutive in six patients and skipping in three patients. In
the forty-three patients of SF group, nine patients (20.9%) were
solitary malformation, and thirty-four patients (79.1%) were
multiple malformations. The thirty-four MM patients included 27
patients of consecutive malformations, 2 patients of skipping
malformations, and 5 patients of consecutive and skipping
malformations. In MT group, six patients (11.5%) were solitary
malformation and 46 patients (88.5%) were multiple
malformations. These 46 MM patients included 24 cases of
consecutive malformations, 4 cases of skipping malformations,
and 18 cases of consecutive and skipping malformations.
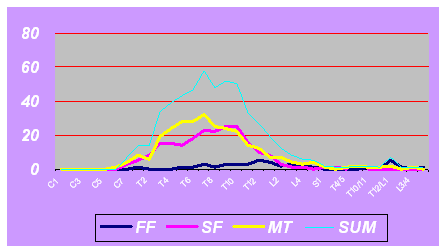
Fig. 1. The frequencies of each involved segment. If the total
segments were more than the normal, the malformed segment was
named by the adjacent two vertebrae. For example, the
hemivertebra between T4 and T5 was named T4/5.
The anomalies of both the anterior component and the posterior
components were the commonest pattern in vertebral deformities.
The proportions of AP malformation were 32.1%, 72.1%, 88.5%, and
69.9% in FF group, SF group, MT group and the series
respectively. The solitary posterior malformation was rare in
our series and all the 3 patients were in SF group. The majority
in FF group was the solitary anterior malformation and the ratio
was 67.9%.
Formation failure included hemivertebra, wedge vertebra, and
butterfly vertebra. In our series, hemivertebra was found in 51
patients (30 in MT group and 21 in FF group), wedge vertebra in
24 patients (16 in MT group and 8 in FF group), and butterfly
vertebra in 9 patients (all in MT group).
In FF group, anomaly of posterior components was not found in 8
patients with wedge vertebra and 11 patients with hemivertebra.
Malformation of posterior
components was found in only 9 of 21 (42.9%) patients with
hemivertebra. In SF group, segmentation failure of posterior
components was found in 33 of 43 (76.7%) patients. In MT group,
formation failures of posterior components were observed in 21
patients (40.4%) and segmentation failure of posterior
components were found in 38 patients (73.1%).
Intraspinal Abnormality
Intraspinal abnormality was found in 31.7% (n=39) of these patients,
including diastematomyelia in 12 patients, tethered cord in 1
patient, combination of diastematomyelia and tethered cord in 21
patients, syringomyelia in 2 patients, combination of
diastematomyelia and syringomyelia in 2 patient and combination
of syringomyelia and tethered cord in 1 patient. There were 26
females and 13 males (female: male ratio 2:1). Intraspinal
abnormality was present in 3.6% (n=1) of the patients with
formation failure (n=28), in 51.2% (n=22) of the patients with
segmentation failure (n=43), and 30.8% (n=16) of the patients of
mixed type (n=52) (Table 2).
Table 2. Intraspinal anomaly in three groups
|
Anomaly |
Total |
Intraspinal anomaly |
|
FF |
28 |
1(3.6%) |
|
SF |
43 |
22(51.2%) |
|
MT |
52 |
16(30.8%) |
|
Total |
123 |
39(31.7%) |
Diastematomyelia was present in total 35 patients. The levels of
diastematomyelia could be T2-S2 and was showed in detail in
Table 3. The length of diastematomyelia ranged from 1 to 14
vertebrae (mean 5.3 vertebrae). The levels of diastematomyelia
were thoracic segments in 5 patients, lumbar segments in 8
patients, thoracic and lumbar segments in 21 patients, and
lumbar and sacral segments in 1 patient. In these patients the
diastematomyelia was not always at the site of spinal deformity,
whereas in some patients it was in the region distant from the
site of the malformed vertebrae. The majority of
diastematomyelia were consecutive in our series and the
diastematomyelia was found to be skipping in only two cases
(Table 3, No.17 and 19). Osseous septum was identified in 6 of
35 patients (17.1%).
Table 3. The levels of diastematomyelia and malformed vertebrae in 35
cases.
|
NO. |
Sex |
Age(years) |
Levels of diastematomyelia |
Levels of malformed vertebrae |
|
1 |
M |
16 |
T6-11 |
T6-10 |
|
2 |
F |
11 |
T12-L1 |
T3-6,T11-L1 |
|
3 |
F |
13 |
T11 |
T3-10 |
|
4 |
F |
14 |
T7-12 |
T3-4,T7-10 |
|
5 |
M |
16 |
T11-L1 |
C7-T8 |
|
6 |
F |
21 |
T2-L1 |
T8-11 |
|
7 |
F |
10 |
T2-12 |
T7,T8-9 |
|
8 |
F |
12 |
L1-3 |
C7-T2,T7,10 |
|
9 |
F |
12 |
L1 |
T9-10 |
|
10 |
F |
12 |
T6-8 |
T6-11 |
|
11 |
M |
14 |
T11-L1 |
T3-6,T10-L1 |
|
12 |
M |
15 |
T2-L1 |
T3-5,T4-6,T10-12 |
|
13 |
M |
12 |
L2 |
T9-11 |
|
14 |
F |
12 |
T2-L3 |
T2-11 |
|
15 |
F |
13 |
T9-L3 |
T9-10 |
|
16 |
F |
13 |
T12-l2 |
T12-L1 |
|
17 |
F |
14 |
T2-5,L1-2 |
T2-7 |
|
18 |
F |
14 |
T7-L2 |
T8-10 |
|
19 |
F |
17 |
T7-11,L3 |
T7-12 |
|
20 |
F |
19 |
L2 |
T3-4,T6-7 |
|
21 |
M |
22 |
T7-L2 |
T7-11 |
|
22 |
F |
7 |
T6-L3 |
T1-6,T7-8,T9-10* |
|
23 |
F |
12 |
L2 |
T9-10,T11-L1 |
|
24 |
M |
15 |
T12-L3 |
T8-10,T11-L4 |
|
25 |
F |
7 |
L1-3 |
T5-6,T9-12 |
|
26 |
M |
9 |
L1-S2 |
L2-3,L4-S1 |
|
27 |
F |
10 |
L2 |
T3-7,T9,L1-2,L5 |
|
28 |
F |
10 |
T9-L3 |
T8-10,T12 |
|
29 |
M |
11 |
T9-L4 |
T4-5,T7,T8-9,T10-11,T12-L5 |
|
30 |
M |
17 |
L3-4 |
T5-9 |
|
31 |
F |
15 |
T7-L4 |
T1-2,T3-5,T12* |
|
32 |
M |
15 |
T10-L2 |
T12 |
|
33 |
F |
11 |
T12-L2 |
T8 |
|
34 |
F |
15 |
T9 |
T6-11 |
|
35 |
F |
5 |
T8-L1 |
C7-T1,T2-5,T12,L3 |
T1-2,T3-5,T12* means there are segmentation failures between T1-2, and
between T3-5 and there is not segmentation failure between T1-2.
T12 is formation failure
Discussion:
Most of the time, the diagnosis of congenital scoliosis through the AP
and lateral plain films is not difficult. However, to describe
the anomaly accurately is not so easy only based on the plain
films. The low sensitivity of plain films to identify the fusion
and hypoplasia of posterior element makes it impossible to
obtain a all-round view of the anomaly. Plain films alone do not
necessarily give the surgeon a clear understanding of the
malformed anatomy, though they remain the standard for diagnosis
and follow-up of these deformities. Plain films are difficult to
interpret because of the complex nature of the deformity,
superimposed structures obscuring visualization of the anomaly,
and spine rotation. Three-dimension CT scans can showed
additional abnormalities not appreciated on plain films, as
reported by Newton [3], so the advantages of three-dimension CT
scans over the conventional radiography are obvious. We do not
have the evidence that three-dimension CT scans gained before
surgery improved the surgical outcome or decreased the
occurrence of complications, but they can help the surgeons to
have an intimate knowledge of the anomaly during the
intraoperative localization and avoid being confused by the
abnormal anatomy during the procedures. Hedequist and Emans [4]
described that three-dimension CT scans accurately predicted
anterior posterior vertebral anomalies in all cases. Nakajima
and Kawakami et al [5] even put forth a new classification of
hemi-lamina type for formation failure with the aid of
three-dimension reconstructions.
Congenital vertebral anomalies encompass a wide variety of defects.
Moe and co-workers use two basic concepts of pathogenesis in
defining most of the anomalies: defects of segmentation and
defects of formation. Van Schrick and MacEwen modified the
classification on this basis. Tsou et al [6] raised a different
classification system. Their unique classification system was
very inclusive and there were two major types and 27 subtypes.
All these classifications were based on either the anatomic
feature of deformity or the classical embryogenesis and prenatal
developmental pattern of the vertebra. However, it was not
helpful to understand the etiology of congenital vertebral
anomaly if the classification was too simple or too complex.
Vertebral development is a complex process and dozens of genes
have been linked to this process [7.8, 9]. The different
components of vertebra were controlled by different genes during
vertebral development. So formation failure or segmentation
failure, anterior deformity or posterior deformity, and solitary
malformation or multiple malformations are taken into
consideration in our classification for further genetic study.
In this study, all of the patients underwent myelography followed by
CTM and three-dimension reconstructions. The aim of myelography
and CTM was to find the intraspinal deformity. Myelography and
CTM are sensitive to diastematomyelia and tethered cord. In our
series, the diagnosis of syringomyelia was made based on MRI
examination, which was done in only 12 patients before they
presented to our hospital. The incidence of intraspinal anomaly
in our series was 31.7%, which was close to the literature
reports. Bradford et al [10] reported an incidence of 38%, using
MRI, in a series of 42 patients. Prahinski et al[11]found a 30%
incidence of intraspinal anomaly in a series of 30 patients.
Belmont et al [12] reported a 35% incidence of intraspinal
anomaly in 106 patients with isolated congenital hemivertebra
with MRI detection. In their report, these abnormalities
included diastematomyelia, cord tethering, Chiari malformations,
and intradural lipomas. Basu et al [13] reported a 37% incidence
of intraspinal abnormality of 126 patients with congenital
scoliosis with MRI examination. However, McMaster [14] reported
that the incidence of intraspinal deformity was 18.3%. In his
report, 106 patients underwent myelography, and diastematomyelia
was diagnosed in 8 patients on the basis of midline bony spur on
plain radiograph. Blake et al [15] performed myelography on a
series of 108 patients with congenital spinal deformity. They
reported an incidence of 58%. Both of these two reports were
based on myelography and the disparity was great compared with
other reports. One possible explanation was the differences of
sensitivity and specificity between myelography and MRI.
Conclusion:
Three-dimensional reconstructions of computed tomography scans are
helpful to provide all-round view of vertebral anomaly and
reliable foundation of classification in congenital scoliosis.
CTM is useful and sensitive to detect the intraspinal deformity,
especially diastematomyelia and cord tethering. The application
of CTM and three-dimensional reconstructions is necessary to
obtain an understanding of both vertebral anomaly and
intraspinal abnormality before the surgery.
Acknowledgements
The
authors are grateful to Lijuan Zhao for her assistance of
collecting the data in this study.
Reference :
1. McMaster MJ, Ohtsuka K. The natural history of congenital
scoliosis: a study of two hundred and fifty-one patients.
Journal of Bone And Joint Surgery Am 1982;64:1128-47.
2. Winter RB, Moe JH, Eilers VE. Congenital scoliosis: a study of 234
patients treated and untreated. Journal of Bone And Joint
Surgery Am 1968;50:1-47.
3. Newton PO, Hahn GW, Fricka KB, Wenger DR. Utility of
three-dimensional and multiplanar reformatted computed
tomography for evaluation of pediatric congenital spine
abnormalities. Spine 2002;27:844-50.
4. Hedequist DJ, Emans JB. The correlation of preoperative
three-dimensional computed tomography reconstructions with
operative findings in congenital scoliosis. Spine
2003;28:2531-4.
5.
Nakajima A,
Kawakami N,
Imagama S,
Tsuji T,
Goto M,
Ohara T.
Three-dimensional analysis of formation failure in congenital
scoliosis. Spine 2007;32:562-7.
6. Tsou P, M’Yau A, Hodgson AR. Embryogenesis and prenatal development
of congenital vertebral anomalies and their classification.
Clinical Orthopaedics And Related Research 1980;152:211-31.
7.
Giampietro PF,
Blank RD,
Raggio CL,
Merchant S,
Jacobsen FS,
Faciszewski T,
et al. Congenital and idiopathic scoliosis: clinical and genetic
aspects. Clinical Medical And Research 2003;1:125-36.
8.
Rida PC,
Le Minh N,
Jiang YJ.
A Notch feeling of somite segmentation and beyond.
Developmental Biology
2004;265:2-22.
9.
Giampietro PF, Raggio CL, Reynolds CE,
Shukla SK, McPherson E, Ghebranious N, et al.
An analysis of PAX1 in the development of vertebral
malformations.
Clinical Genetics
2005;68:448-53.
10. Bradford DS, Heithoff KB, Cohen M. Intraspinal abnormalities and
congenital spine deformities: A radiographic and MRI study.
Journal of Paediatric Orthoppaedics 1991;11:36-41.
11. Prahinski JR, Polly DW, McHale KA, Ellenbogen RG. Occult
intraspianl anomalies in congenital scoliosis. Journal of
Paediatric Orthoppaedics 2003;20:59-63.
12. Belmont DJ, Kuklo TR, Taylor KF, Freedman BA, Prahinski JR, Kruse
RW. Intraspinal anomalies associated with isolated congenital
hemivertebra: the role of routine magnetic resonance imaging.
Journal of Bone And Joint Surgery Am 2004;86-A:1704-1710.
13.
Basu PS,
Elsebaie H,
Noordeen MH.
Congenital spinal deformity: a comprehensive assessment at
presentation.
Spine
2002;27:2255-9.
14. McMaster MJ. Occult intraspinal anomalies and congenital
scoliosis. Journal of Bone And Joint Surgery Am 1984;66:588-601.
15. Blake NS, Lynch AS, Dowling FE. Spinal cord abnormalities in
congenital scoliosis. Annual Radiology 1986;29:377-9.
|



